Figure 2. Mechanism for regulatory lipids controlling the immune response.
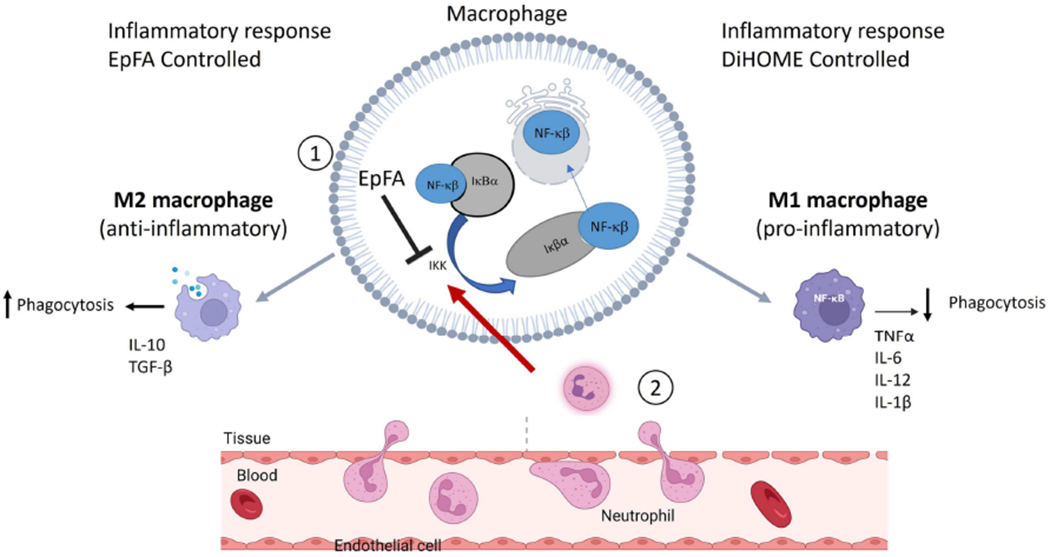
Background: NFκβ is a transcription factor that is regulated by binding to the cellular protein I-kappa-beta-alpha (Iκβα). Binding to Iκβα prevents translocation of NFκβ into the nucleus. Inflammatory cytokines activate IκB kinase (IKK) to degrade Iκβα and release NFκβ, allowing for nuclear translocation and further initiation of inflammatory cytokines (Castro-Alcaraz, Miskolci, Kalasapudi, Davidson, & Vancurova, 2002). Neutrophils are one of the first leukocytes to migrate to the inflammatory site and activate IKK to initiate an inflammatory cascade. These actions result in an inflammatory phenotype (M1 polarization of macrophages). In contract, suppression of NFκβ leads to an anti-inflammatory phenotype (M2 polarization of macrophages). Effects of regulatory lipids on inflammation: 1. EpFA (e.g. EpETrE formed from AA) blocks inflammatory signaling by inhibiting IKK to prevent subsequent nuclear translocation of NFκβ. EpFA preserve M2 polarization and resolution of inflammation, phagocytosis and anti-inflammatory cytokines. (Dai, et al., 2015) {Abdalla, 2023 #3055}. In contrast, DiHOMEs activate neutrophils, signal IKK to degrade Iκβα and allow for NFκβ to initiate an inflammatory cascade. Continued activation by neutrophils induce M1 polarization of macrophages, reduce phagocytosis and promote chronic, dysregulated inflammation. (Lin, et al., 2022). Furthermore, continued signaling from neutrophils will continue the inflammation resulting in inflamed endothelial cells, and disruption of vascular integrity (Martin-Fernandez, et al., 2021). Image adapted from BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates
