Figure 5. ACSS2-generated Ac-CoA promotes protein stability of ALK5.
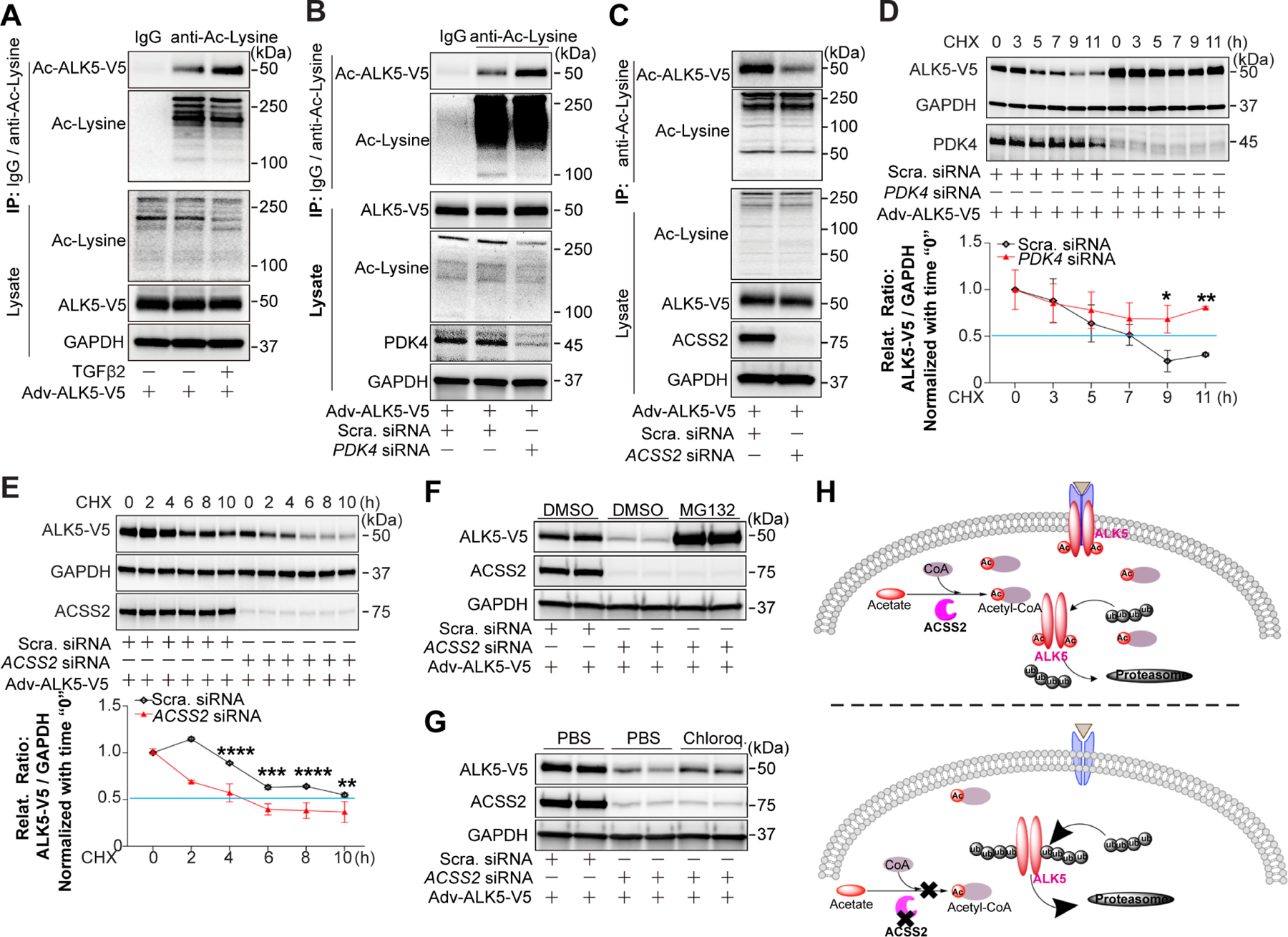
ALK5-V5 was adenovirally overexpressed in HUAECs three days before assays. A, Representative blot of acetylated ALK5-V5 in HUAECs treated or non-treated with TGFβ2 (10 ng/ml in complete EGM-2 medium) for 7 days. Acetylated proteins were captured using agarose beads conjugated with anti-Acetylated lysine antibody, and acetylated ALK5-V5 (Ac-ALK5-V5) was detected using anti-V5 tag antibody. B-C, Blots of Ac-ALK5-V5 in HUAECs transduced with Scramble siRNA or PDK4 siRNA(B) / ACSS2 siRNA(C) separately for 7 days. Acetylated proteins were captured and detected as described above. D-E, Half-life of ALK5-V5 in HUAECs transduced with Scramble siRNA or PDK4 siRNA(D) / ACSS2 siRNA (E). ALK5-V5 was adenovirally overexpressed in HUAECs cycloheximide (CHX, 10 μg/ml) treatment at indicated time points. ALK5-V5 protein level was determined by anti-V5 antibody. F-G, Representative blots showing the regulation of ALK5 degradation in HUAECs. HUAECs were first transduced with Scramble siRNA or ACSS2 siRNA, then ALK5-V5 was adenovirally overexpressed in HUAECs followed by treatment with a proteasome inhibitor MG132(F) or a lysosome inhibitor chloroquine (G). H, Diagram for Ac-CoA-regulated ALK5 acetylation results in increased protein half-life due to decreased proteasomal degradation.
