Fig. 5 |. Model of RXFP1 activation by relaxin-2.
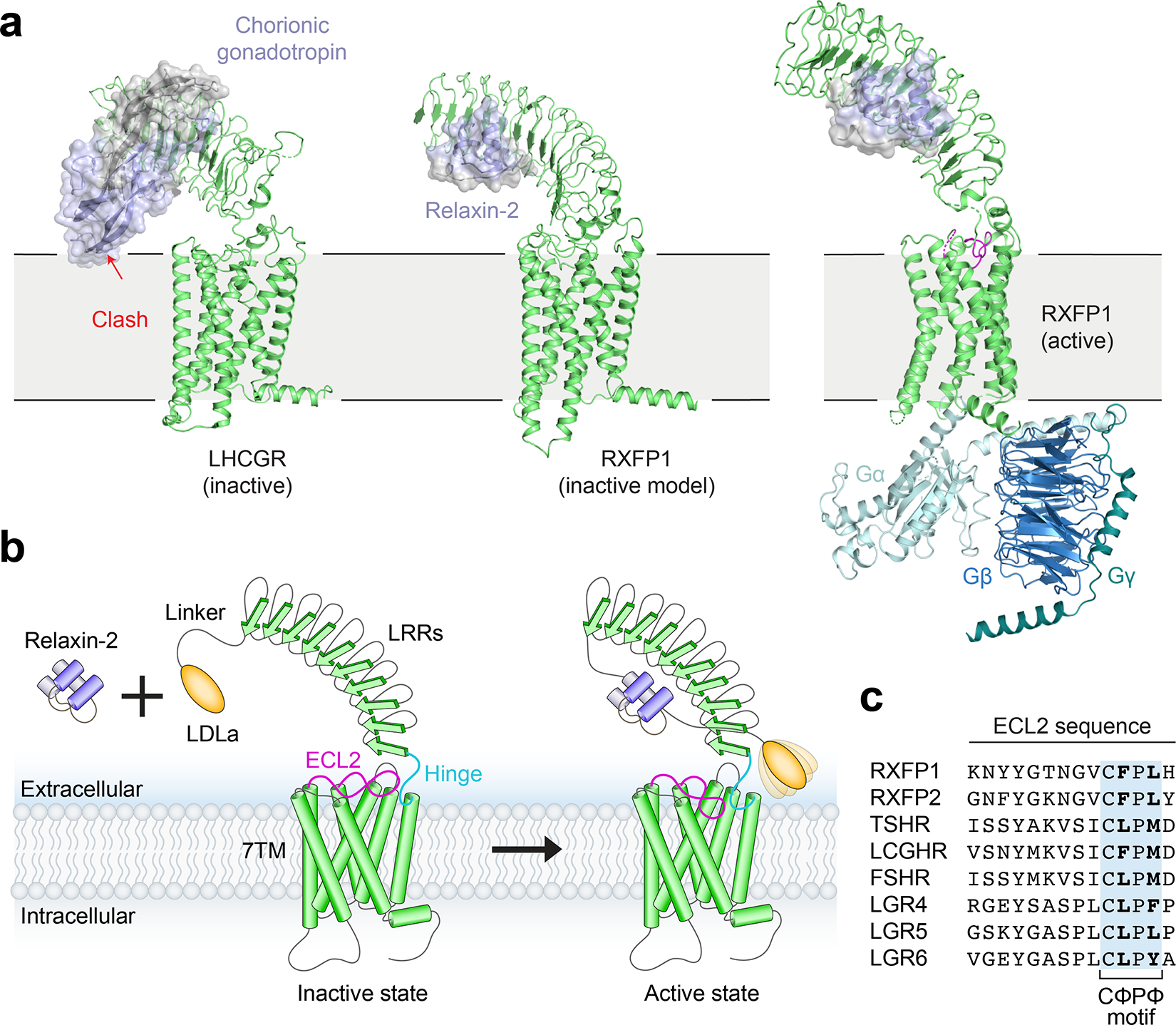
a, Model of LHCGR in the inactive conformation (PDB ID: 7FIJ) bound to the hormone chorionic gonadotropin, showing steric clash with the membrane that induces activation8. In contrast, a similar potential conformation of RXFP1 would easily accommodate bound relaxin-2 (generated from alignments of inactive-state AlphaFold2 models of RXFP1’s LRRs and 7TM to PDB 7FIJ). A hybrid model of active-state RXFP1 is also shown for comparison, based on our 7TM domain structure, cryo-EM maps, and AlphaFold2 model of the LRRs with docked relaxin-2 hormone. The hybrid model uses Gγ2 from PDB 3SN610. b, The inactive state of RXFP1 is characterized by inhibitory interactions between ECL2, the hinge region, and the LRRs that prevent receptor activation, although the exact conformation of RXFP1 domains in this state remain undefined. Relaxin-2 binds to the concave side of the LRRs, away from the 7TM domain. Relaxin-2 binding leads to reorganization of the LDLa/hinge/ECL2 interface into the active state, allowing residues in both the hinge region and ECL2 to induce receptor signaling. The LDLa module is potentially mobile in RXFP1’s active state, based on the lack of density for this domain in our cryo-EM map. The temporal sequence of conformational changes remains to be determined. c, Sequence alignment of human LGRs showing the conserved CΦPΦ motif in ECL2.
