Table 1:
List of HBV capsid assembly modulators in clinical development.
| HBV CAM/Class | Company | Clinical trial phase | Structure | HBV DNA log10 IU/mL reduction (in vivo) | HBsAg/HBeAg log10 IU/mL reduction | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|
| Morphothiadin (GLS4)/I | HEC Pharma | II |
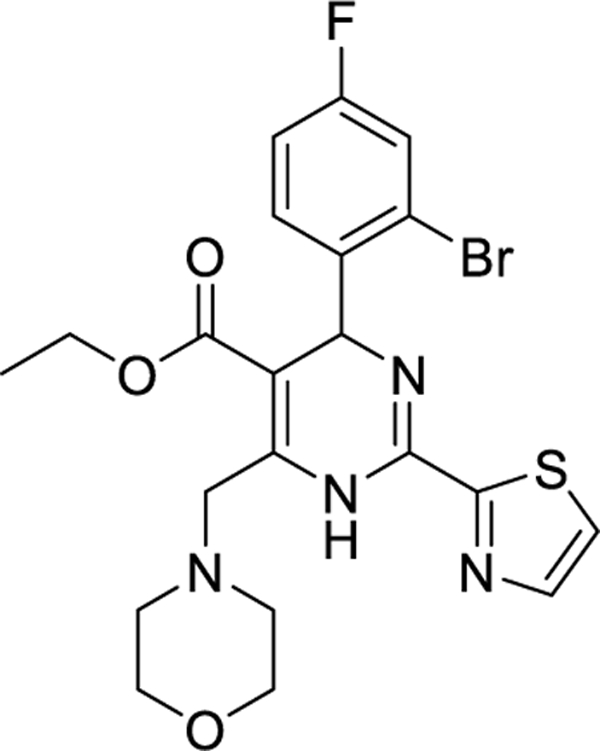
|
1.4 and 2.1 at 28 days of
monotherapy. 5.0 at 12 weeks GLS4/ritonavir combined with ETV |
Minimal with monotherapy or combined with
ETV. 1.59 pgRNA reduction combined with ETV. |
NCT03638076
NCT03662568 NCT04147208 |
| Bersacapavir JNJ 56136379/II | Janssen | II |
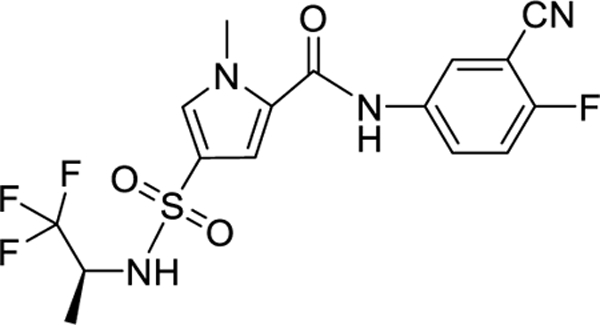
|
5.5 and 5.9 at week 24 of monotherapy or
combined with TDF. Monotherapy resulted in T33N viral resistance. |
Minimal with monotherapy or combined with
TDF. Maximal individual HBsAg and HBeAg reductions were 1.28 and 1.8 at week 24 when combined with TDF in patients with the most pronounced HBV DNA declines at week 24. |
NCT04208399
NCT04474210 NCT02933580 NCT02662712 NCT02662712 NCT03982186 NCT04129554 NCT04439539 NCT04667104 NCT04585789 |
| QL-007 | Qilu Pharmaceutical | II | Not disclosed | Not available | Not available |
NCT03770624
NCT03244085 NCT04157699 NCT04157257 |
| EDP-514/II | Enanta Pharma | I | Not disclosed | > 4 (in chimeric mice). 2.9 to 3.5 after 28 days treatment in CHB, non-cirrhotic, viremic, HBeAg (+). | HBV RNA reduction (up to 2.9) |
NCT04470388
NCT04008004 NCT04783753 NCT04971512 |
| ABI-H3733/II | Assembly Biosciences | I | Not disclosed | 3.1 | Not available |
NCT05414981
NCT04271592 |
| Canocapavir (ZM-H1505R)/ Pirazole | Zhimeng Biopharma | I |
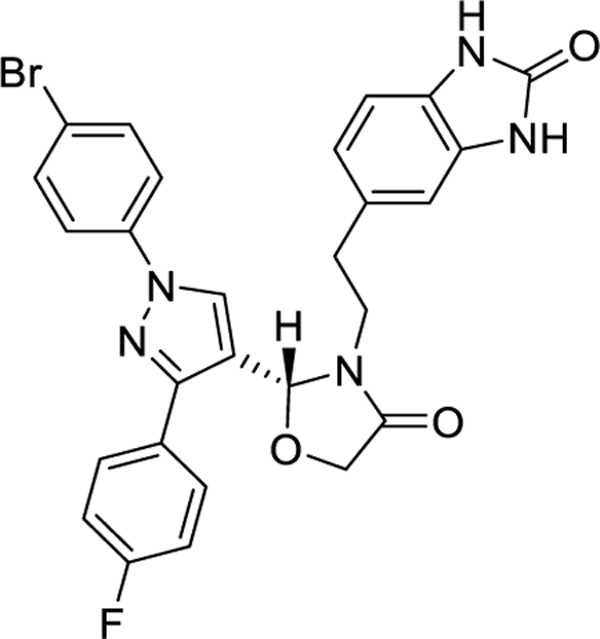
|
2.7 with monotherapy at 200 mg (28 days treatment) | No reduction of surface or e
antigens. pgRNA: 2.3 log10 copies/mL reduction. |
NCT05484466
NCT05470829 NCT04220801 |
| ALG-000184/II | Aligos Therapeutics | I | Not disclosed but related to GLP-26 | 4.2 | 0.8 HBsAg reduction. | NCT04536337 |
| VNRX-9945/I | Venatorx | I-terminated | Not disclosed | EC50 = 3–31 nM* | Not available | NCT04845321 |
| KL060332/I | Kelun-Biotech Biopharmaceuticals Co | I | Not disclosed | ~3 in HBV mouse model | Reduced surface and e antigens (>1.5) | Not available |
| ABI-4334/II | Assembly Biosciences | I | Not disclosed | EC50 = 0.5 nM* | pgRNA reduction*. cccDNA-EC50 = 3.1nM* (PHH). |
NCT05569941 |
In vitro; ETV, entecavir; pgRNA, pre-genomic RNA; TDF, tenofovir disoproxil fumarate; CHB, chronic hepatitis B; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; cccDNA, covalently closed circular DNA; EC50, half maximal effective concentration; PHH, primary human hepatocytes.
