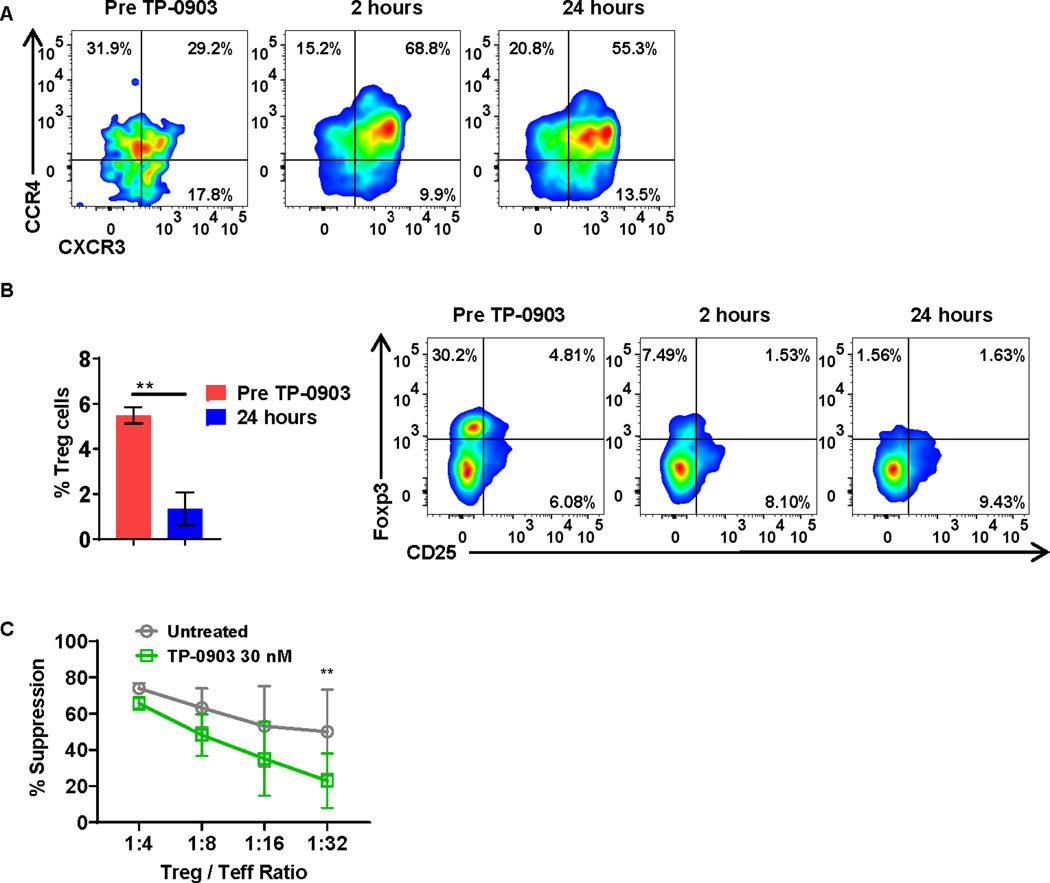
Figure 4: T-cell phenotype is altered following treatment with TP-0903 in a phase I clinical trial for patients with solid tumors (NCT02729298).
A, Peripheral blood-derived Th2 cells (CD4+CCR6−CXCR3−CCR4+) from patients were analyzed before and one day after treatment with TP-0903. Representative flow plot of 3 biological replicates is shown. B, Peripheral blood-derived Tregs (CD4+CD25+Foxp3+) from patients were analyzed before and one day after treatment with TP-0903. ** p<0.01, t-test, n=3 biological replicates. C, Tregs and carboxyfluorescein succinimidyl ester (CFSE) stained effector T cell (Teff) cells were co-cultured at the indicated ratio and co-cultured for 4 days in the presence or absence of 30 nM of TP-0903. At the end of the co-culture, the percent suppression of Teff was calculated using flow cytometry. ** p<0.01, two-way ANOVA, n=3, biological replicates.