Figure 1. Inflammasome activation induces pyroptosis.
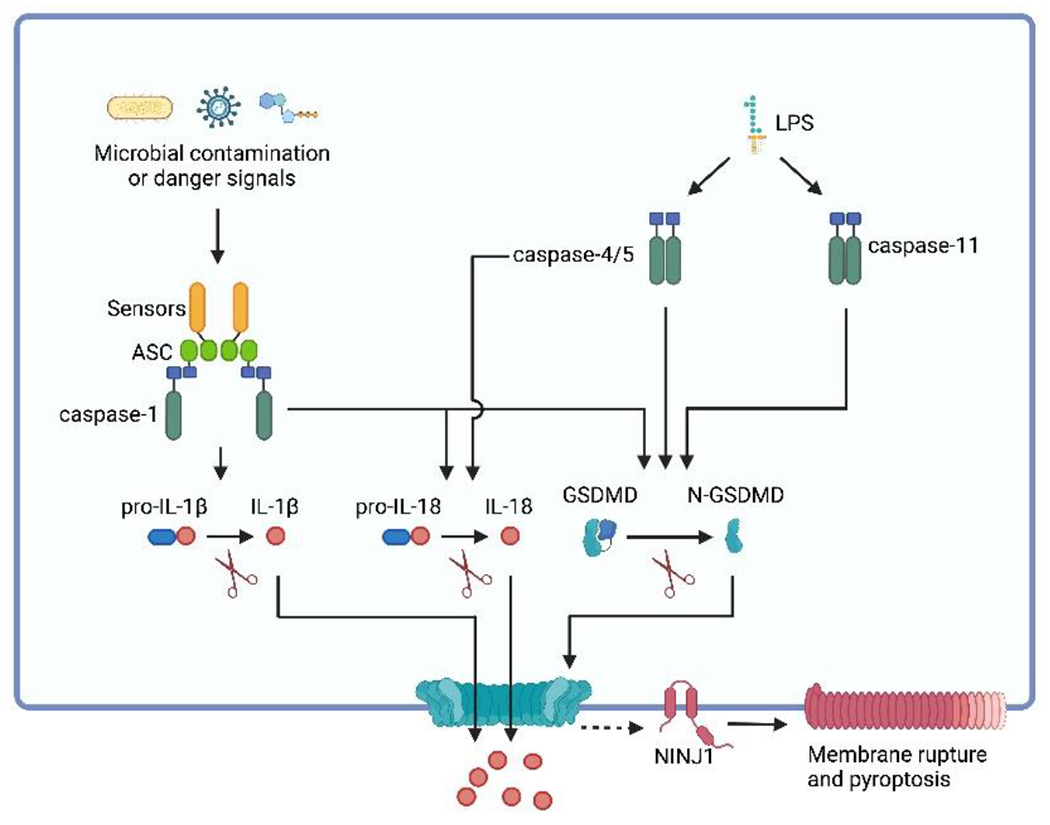
Inflammasomes are multiprotein complexes located in the cytosol that trigger the activation of caspase-1. Typically, an inflammasome comprises an inflammasome sensor and often the protein adaptor ASC. Inflammasome sensors are responsible for detecting various cellular disturbances, including microbial contaminants or danger signals. Upon polymerization, inflammasome sensors often recruit ASC, leading to the formation of ASC specks. Inflammasomes either directly or via ASC recruit pro-caspase-1 and trigger its activation. Once activated, caspase-1 cleaves GSDMD into its active form, N-terminal GSDMD (N-GSDMD). N-GSDMD polymerizes and forms pores in the plasma membrane, resulting in pyroptosis. NINJ1, a transmembrane protein, facilitates plasma membrane rupture in pyroptosis and other types of lytic cell death. Furthermore, caspase-1 also cleaves pro-IL-1β and pro-IL-18, generating active IL-1β and IL-18, respectively. These pro-inflammatory cytokines can be released from the GSDMD pores, along with other inflammatory cellular components. In parallel, caspase-4/5/11, are activated by cytosolic LPS. This activation leads to the cleavage of GSDMD, ultimately inducing pyroptosis. Notably, caspase-4/5 but not caspasae-11 can cleave pro-IL-18 into its mature form156.
