Figure 4. Activation mechanisms of gasdermins.
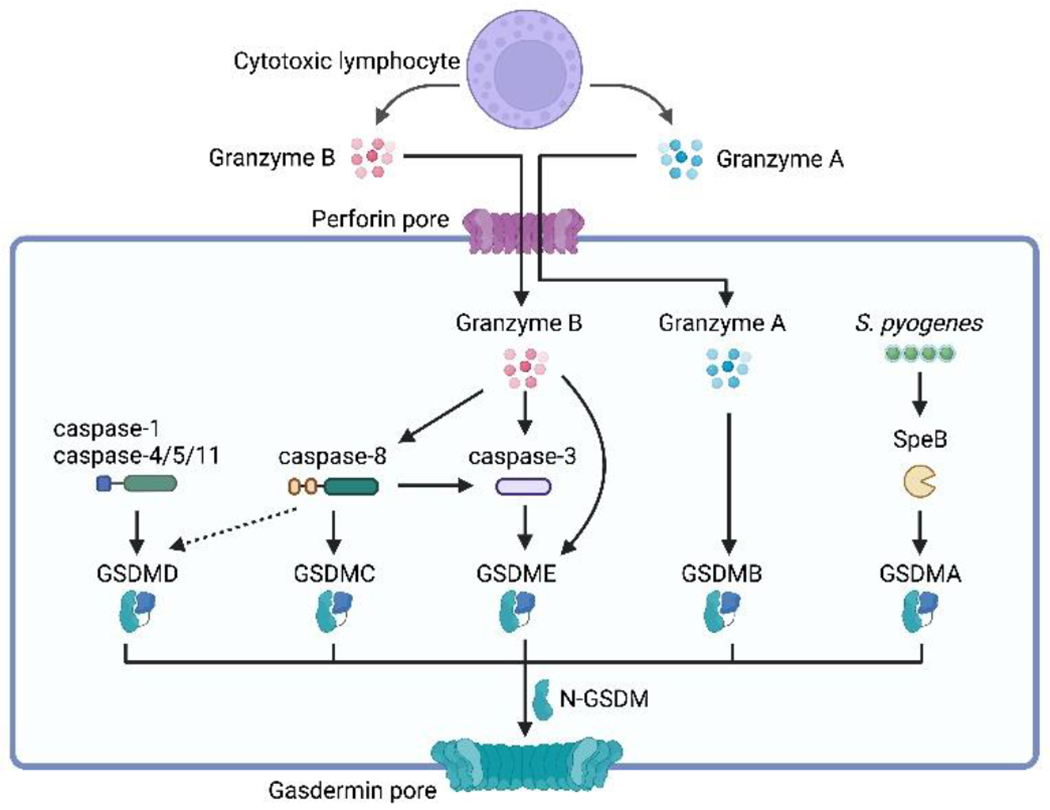
Pyroptosis is triggered through the cleavage of gasdermin proteins, which involves both inflammasome-dependent and inflammasome-independent pathways. Gasdermin D (GSDMD), the prototype of gasdermins, can be activated by inflammatory caspases, including caspase-1/4/5/11, through the inflammasome-dependent pathway. Gasdermins can also divert apoptotic signaling towards pyroptosis through their cleavage. Caspase-8, traditionally known as an initiator of apoptosis, can induce pyroptosis by cleaving GSDMD under specific conditions. Caspase-8 can also cleave GSDMC, which is primarily expressed in epithelial tissues, leading to pyroptosis. Caspase-3 cleaves GSDME, transitioning from caspase-3-mediated apoptosis to pyroptosis. Furthermore, granzyme B, produced by cytotoxic lymphocytes, can directly cleave GSDME, resulting in pyroptosis independent of caspase-3. Granzyme A, another protease produced by cytotoxic lymphocytes, activates GSDMB, triggering pyroptosis in target cells. In addition to host-derived proteases, pathogen-derived factors can also activate gasdermins. For instance, the cysteine protease virulence factor SpeB produced by Streptococcus pyogenes cleaves GSDMA, inducing pyroptosis in keratinocytes.
