Figure 12. Summary model for regulation of MurZ and MurA enzymatic activities by StkP-mediated phosphorylation in S. pneumoniae D39.
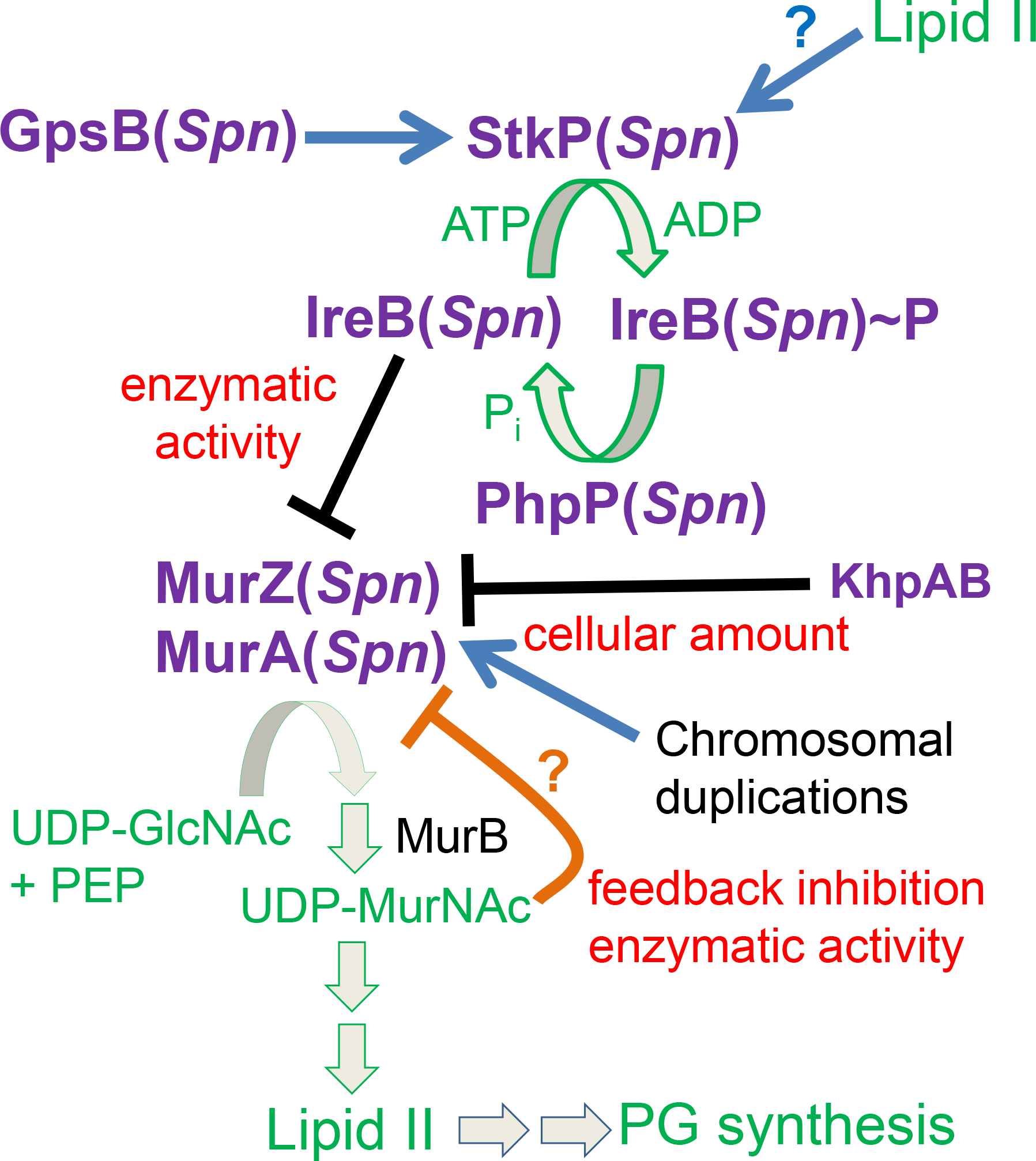
GpsB(Spn) and possibly other ligands, such as Lipid II, stimulate the phosphorylation of a negative regulator of MurZ(Spn) and MurA(Spn) enzymatic activity, but not their cellular amounts, in the first committed step of Lipid II synthesis for PG synthesis. By genetic criteria presented here, the negative regulator is unphosphorylated IreB(Spn). Phosphorylated IreB(Spn)~P does not bind to MurZ(Spn) or MurA(Spn), resulting in full enzymatic activity in pneumococcal cells growing exponentially in rich media. The absence of GpsB(Spn) significantly reduces phosphorylation of IreB(Spn) leading to inhibition of MurZ(Spn) and MurA(Spn) enzymatic activities and no growth. This inhibition can be relieved by inactivation of the cognate PhpP protein phosphatase, which allows residual phosphorylation to IreB(Spn)~P. The absence of the StkP protein kinase and the need for protein phosphorylation in pneumococcal cells growing exponentially in rich media can be suppressed by inactivation or absence of the IreB(Spn) negative regulator, by amino-acid changes in a regulatory domain of MurZ(Spn), which is enzymatically predominant over MurA(Spn), or by overexpression of murZ(Spn) or murA(Spn) in spontaneous chromosomal duplications. Moderate MurZ(Spn) overproduction sufficient to suppress the absence of StkP also occurs in the absence of the KhpAB RNA-binding protein, which also negatively regulates FtsA amount. This pathway provides a positive feedback loop, such that cells growing rapidly in rich media produce Lipid II, which may activate StkP(Spn) to fully phosphorylate IreB(Spn) and maximize MurZ(Spn) and MurA(Spn) enzymatic activities for the production of even more Lipid II for PG synthesis. Evidence for the direct interaction between unphosphorylated IreB(Spn) and MurZ(Spn) will be presented elsewhere (Merrin Joseph, unpublished result). Structures predicted by AlphaFold v2.0 also suggest that MurZ(Spn) and MurA(Spn) enzymatic activity is subject to negative pathway feedback inhibition by binding of UDP-MurNAc (UDP-N-acetylmuramic acid) near the catalytic sites of the enzymes (Mizyed et al., 2005, Schonbrunn et al., 2000). See text for additional details.
