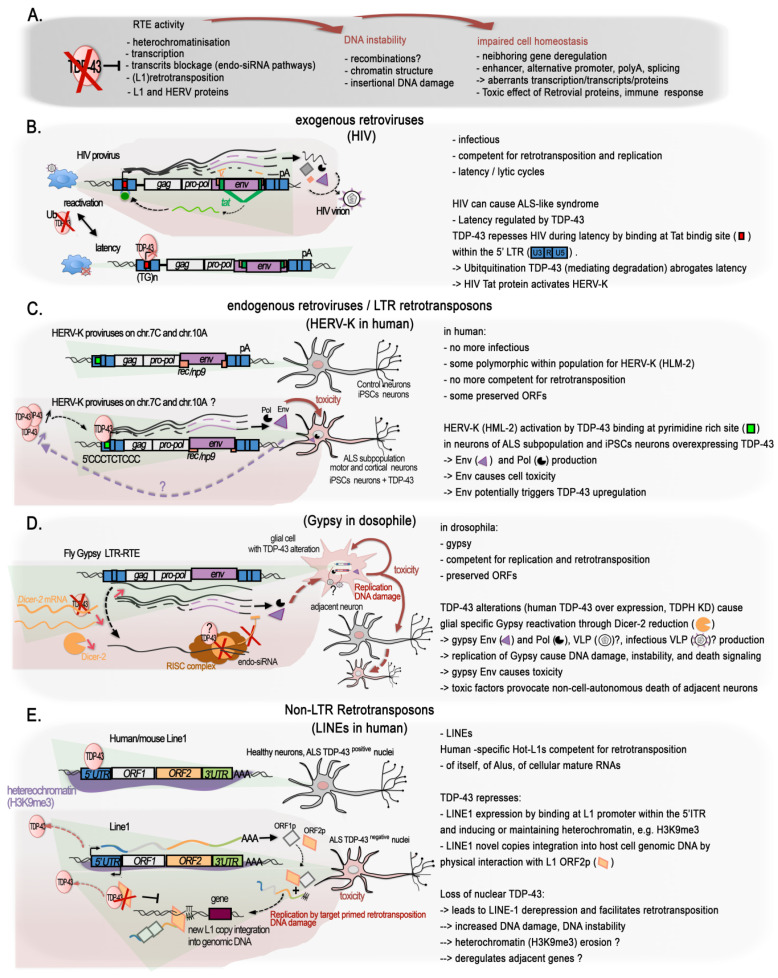
Figure 3.
TDP-43 connections to RTE detrimental action in ALS. (A) Overview of the general consequences of TDP-43 alterations in DNA stability and cellular homeostasis due to RTE inhibition failure. (B–E) TDP-43 impact on ALS through the misregulation of RTE. In the cartoons, regulatory sequences (promoter regions), i.e., LTRs in exogenous and endogenous ERVs, and 5′UTR in Lines, are in blue; retroviral gag, pro, and pol genes produced from the same (polycistronic) transcript are in white; and the env gene produced by an alternatively spliced transcript is in purple. ORFs for accessory proteins (Tat in HIV, rec and np9 or rec in HERV-K) are depicted. Specificities of each class/specific elements and impact on ALS are listed on the right of each cartoon. (B) TDP-43 is able to repress HIV-1 provirus activation. TDP-43 binds to the TAR binding site within the 5′LTR R region and represses transcription. This binding was shown to impede the binding of TAR RNA and Tat-activating protein. A reduction in TDP-43 binding (by ubiquitination and proteosomal degradation) can reverse HIV-1 provirus latency, potentially leading to the production of infectious viral particles (HIV virion). It is known that HIV-1 can promote ALS-like symptoms. HIV can also activate HERV-K elements, notably via Tat [155]. (C) TDP-43 overexpression binds to and activates specific HERV-K HML-2 provirus(es), producing the toxic env glycoprotein HERV(HML-2). The HERV-K proviruses found activated in ALS cases (ALS neurons shows immunoreactivity for HERV-K Env) could be HERV-K C7-C and HERV-K C10-A, which are polymorphic proviruses in the human population. TDP-43 binds to the LTR5Hs sequence at a polypyrimidine track in the U3 region (5′-CCCTCTCCC-3′) with high affinity and is able to activate the LTR-promoted transcription. Conversely, HERV-K Env potentially triggers TDP-43 upregulation. (D) In Drosophila, the failure of TDP-43 to indirectly repress Gypsy retrovirus, a family of endogenous LTR-retrotranposon, leads to cell autonomous and non-autonomous toxicity. The family contains copies with preserved ORFs, capable of retrotransposition and replication. TDP-43 alterations (hTDP-43 overexpression or fly TDP-43 homologue TDPH null) induce the activation, specifically in glial cells, of Gypsy copies. It is not clear if replication involves infectious or non-virus-like particles (VLP). Mechanistically, TDP-43 binds to and positively regulates Dicer-2 (Dicer in the human) mRNA and protein. Dicer-2 in the RISC complex controls Gypsy and other RTE activity via the endo-siRNA pathway, inhibiting or impeding transduction by promoting mRNA degradation. Lack of TDP-43 by reducing Dicer-2 levels impedes endo-siRNA-mediated control (see text for more detail). (E) TDP-43 nuclear loss in the neurons of ALS patients induces L1 expression. TDP-43 represses human L1 by at least two mechanisms: (i) by binding at the 5′UTR promotes and maintaining L1 heterochromatinization; and (ii) by interacting with the ORF2p in cases of L1 retrotransposition, inhibiting the pasting of new copies into the host genome.