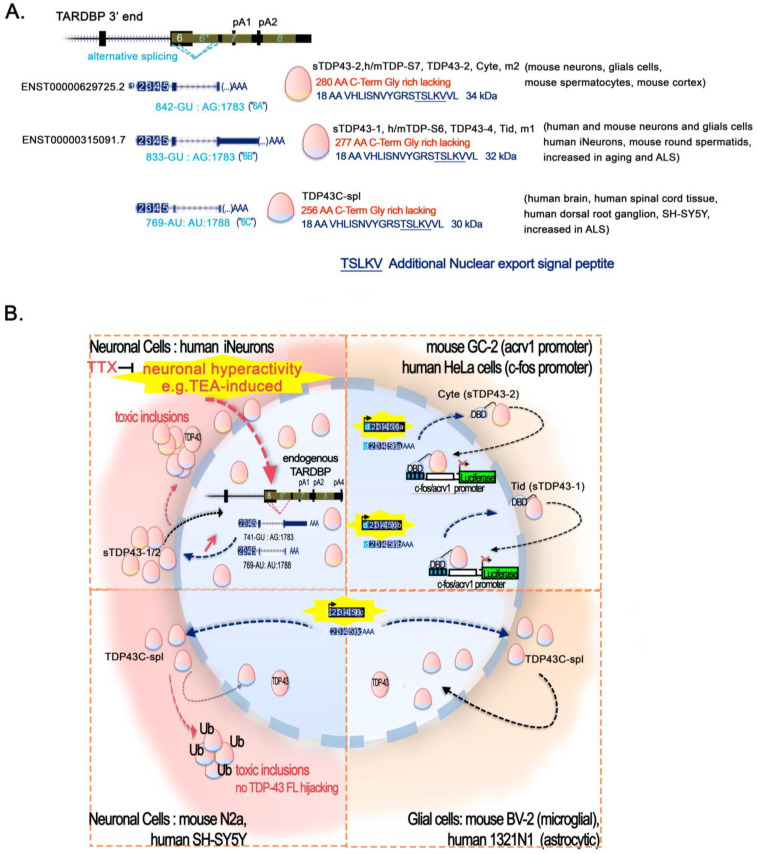
Figure 4.
Short-TDP-43 isoforms: alternative splicing and context-specific fate. (A) Alternative splicing leading to short-TDP-43 proteins in brain. Transcripts resulting from various splicing of the alternative intron 6 (*) within TDP-43 pre-mRNA and translated into different short-TDP-43 proteins readily observed in mouse and human brains are depicted: ENST00000629725.2 encoding sTDP43-2 (also called hTDP-S7, mTDP-S7, TDP43-2, m2, or Cyte); ENST00000315091.7 encoding sTDP3-1 (also called hTDP-S6, mTDP-S6, TDP43-4, m1, or Tid). Both are highly conserved at the transcript and protein levels and the recently identified transcript producing TDP43C-spl. Note that the TSS and the TTS of the transcripts of these isoforms have not been validated, and it is not clear if they hold the 3′UTR TDPBR needed for autoregulation via TDP-43 FL. Short TDP43 isoforms-specific intron borders with splice donors (SD) and acceptors (SA) are indicated under each transcript in blue, with numbering given relative to the CDS +1. SD and SA are all located within TARDBP exon 6, eliminating the majority of exon 6. The sTDP43 protein isoforms contain at least the first 256AA and up to the first 280AA of TDP-43 (indicated in red). They contain the N-Term NLS, the RRM 1 and 2, and the NES, but not the C-terminal glycine-rich region in the TDP-43 protein. They gain an alternative 16 to 18AA, forming a unique C-end term VHLISNVYGRSTSLKVV, and sheltering a second nuclear export sequence (NSE) consisting in TSLKV. sTDP43-1, sTDP43-2, and TDP43C-spl have a mass of about 34 kDa, 32 kDa, and 30 kDa, respectively, and have been spotted in several vulnerable zones of the CNS both in normal and ALS patients, as well as in mouse male germ cells, as indicated between brackets. (B) Cell type sensitivity to high expression of short TDP proteins. Abnormal localization and ubiquitination of short TDP-43 aggregates leading to toxic inclusions are a pathological hallmark of neurons and glia in neurodegenerative diseases. Note the repressive potential of the mouse short TDP-43 isoforms Cyte and Tid (sTDP43-2 and sTDP43-1, respectively) on Acrv1 and c-fos promoters in GC-2 and Hela cells. The tethering of Cyte or Tid to a reporter plasmid using Gal4 DNA binding domain (DBD) fused to TDP-43 at Gal4 binding sequences (blue boxes) represses c-fos or Acrv1 promoters-induced luciferase expression, as does TDP-43 FL in the same conditions (up right frame). In neurons (left frames), human and mouse sTDP43-1 and 2 are present either in the nucleus or in the cytoplasm (soma and axons) or in both. They are upregulated by age and in response to increased neuronal activity (e.g., TEA in human iNeurons, or bicuculline in rodent primary mixed cortical neurons), and are conversely downregulated by TTX that abolishes neuronal activity. When overexpressed, they form insoluble aggregates in the cytoplasm able to sequester full-length TDP-43, leading to nuclear clearance of endogenous TDP-43 and neurotoxicity. TDP-43C-spl is observed in the cytoplasm of the human spinal cord, brain tissue, and dorsal root ganglia. TDP-43Cspl overexpression in neuronal cell lines convey their delocalization to the cytoplasm, where they form toxic ubiquitinated aggregates. In astrocytoma and microglia cell lines, TDP-43Cspl is not delocalized to the cytoplasm and localizes in interchromatin granule clusters (speckles) in the nucleus. TDP-43 is not recruited to the TDP43C-spl aggregates, but keeps its nuclear localization (bottom right frame).