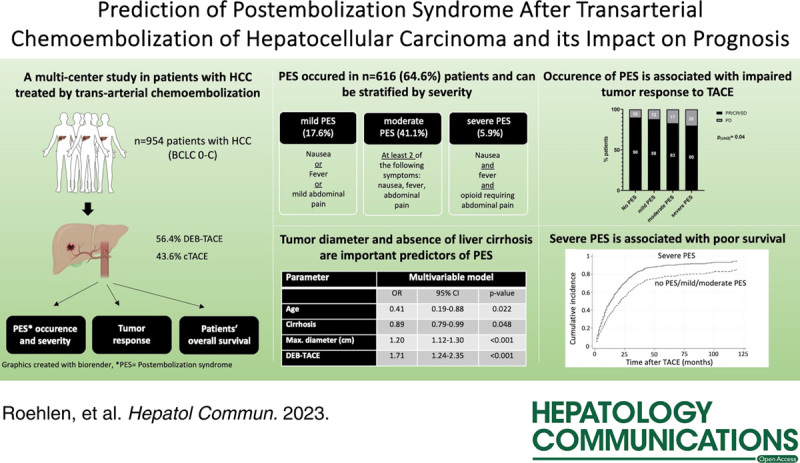
Abstract
Background:
Postembolization syndrome (PES) represents the most frequent complication after transarterial chemoembolization (TACE) in patients with HCC. Given the vague definition as a symptom complex comprising abdominal pain, fever, and nausea, PES is diagnosed in heterogeneous patient cohorts with symptoms ranging from mild pain to severe deterioration of their general condition. This study aimed to evaluate predictive factors and the prognostic impact of PES with regard to different severity grades.
Methods:
A total of 954 patients treated with TACE for HCC at the University Medical Centres Mainz and Freiburg were included in this study. PES disease severity was graded as mild, moderate, or severe according to a predefined combination of symptoms. Logistic regression models were used to identify independent predictors of PES. The prognostic impact of PES was evaluated by competing risk analyses considering liver transplantation as a competing risk.
Results:
PES occurred in 616 patients (64.5%), but only 56 patients (5.9%) had severe PES, defined as moderate to severe abdominal pain requiring opioids in combination with fever and nausea. The largest tumor diameter was the strongest independent predictor of PES (OR = 1.21, 95% CI = 1.13–1.28), and severe PES (OR = 1.23, 95% CI = 1.14–1.33, p < 0.0001). Presence of liver cirrhosis was protective against PES (OR = 0.48, 95% CI = 0.27–0.84, p = 0.01). Furthermore, PES was independently associated with an impaired disease control rate (OR = 0.33, 95% CI = 0.16–0.69, p = 0.003) and severe PES with poor overall survival (subdistribution HR = 1.53, 95% CI = 0.99–2.36, p = 0.04).
Conclusions:
Tumor size and absence of liver cirrhosis are predictors of severe PES and associated with impaired prognosis in HCC patients after TACE.
INTRODUCTION
HCC is the third leading cause of cancer-related death, with increasing incidence worldwide.1,2 Transarterial chemoembolization (TACE) is the most widely applied locoregional treatment approach for patients not eligible for curative treatments.3,4 TACE can improve survival and bridge or downstage patients to curative treatment options.3 As a minimally invasive intervention, TACE can be performed with 2 alternative techniques. Conventional TACE (cTACE) includes selective injection of 1 or more emulsified chemotherapeutic agents into the tumor-feeding arteries and subsequent embolization. Drug-eluting bead (DEB-) TACE is characterized by the use of microspheres loaded with chemotherapeutic agents, which are selectively injected in tumor arteries, potentially followed by further embolization.5 The technique, angiography equipment, and technology have evolved over the last few years, with TACE regularly being performed in a superselective fashion, reducing the risk of severe complications, such as i.p. hemorrhage, cholecystitis, and deterioration of liver function.6–8
Postembolization syndrome (PES) is a common adverse event, even after superselective TACE.6,9 Given the vague definition as a syndrome characterized by abdominal pain, fever, and nausea/vomiting, reported incidences of PES range from 6.2%10 to >80%.9,11 Despite its frequent occurrence, the prognostic relevance and predictive factors remain controversial.11–14 In this study, we aimed to evaluate predictive factors for the occurrence of PES, as well as its prognostic relevance with regard to different severity grades.
METHODS
Patient cohort
A total of 960 patients who underwent first TACE for HCC treatment between September 2005 and May 2020 at the University Medical Centers Mainz (n = 602) and Freiburg (n = 358) were screened for this retrospective observational study. Patients with previous TACE treatment were not considered in these analyses. Six patients with CHILD C liver cirrhosis (BCLC stage D) were excluded since indication for locoregional therapy was based on highly individual tumor board decisions leading to a final overall patient cohort of 954 patients. Cirrhosis was diagnosed by pathognomonic findings in ultrasound examinations or cross-sectional images and typical laboratory characteristics and/or liver biopsy. HCC was staged using the Barcelona Clinic Liver Cancer (BCLC) classification. Diagnosis of HCC was made according to the current guidelines mainly by imaging (CT or dynamic contrast-enhanced MRI) when lesions showed the typical arterial phase hyperenhancement and portal venous and/or delayed washout or by biopsy.3,15
All transarterial interventions were performed by experienced board-certified interventional radiologists. During the procedure, all patients received an antiemetic drug (ondansetron, granisetron, or tropisetron). Based on the individual decision of the interventionalist, 56.4% of the patients received DEB-TACE and 43.6% cTACE. After the procedure, patients were monitored on medical wards of the University Medical Centers Mainz and Freiburg for a minimal follow-up time of 48 hours, and postprocedural symptoms and complications were surveyed by medical professionals. Temperature was measured at least twice per day. Symptoms after this postinterventional time period were not considered in the analysis. This study includes retrospective analyses of patients’ clinical data. All patients provided written informed consent for the medical data to be recorded prior to inclusion to this study. The study was approved by the local ethics committee of the University Mainz (EK/2020-15304) and the University Medical Center Freiburg (EK355/20) and was conducted in accordance with the Declaration of Helsinki and Istanbul.
Definition of PES and severity grades
PES was defined as a syndrome that occurred 1–3 days after TACE with patients showing at least one of the following symptoms: fever >38.5 °C, nausea and/or vomiting, and abdominal pain requiring administration of analgetics.9,13,14 To categorize PES into different severity grades, we introduced a scoring system based on variable points for the presence of each syndrome-defining symptom. Using this scoring system, all patients were subgrouped into individuals without PES (0 points, n = 344 patients), mild PES (1 point, n = 166), moderate PES (2–3 points, n = 394), and severe PES (4 points, n = 56) (Table 1).
TABLE 1.
Classification of PES severity
| Points | ||
|---|---|---|
| Symptom | 1 | 2 |
| Nausea | Nausea with or without vomiting | — |
| Fever | Fever >38.5°C | — |
| Abdominal pain | Mild pain requiring eventual administration of analgesics | Moderate to severe pain requiring opioids |
| No PES = 0 points; mild PES = 1 point; moderate PES = 2–3 points; severe PES = 4 points | ||
Abbreviation: PES, postembolization syndrome.
Evaluation of tumor response and survival
All patients were followed up for a median 51 months. One to 3 months after TACE, the tumor response was assessed by CT or MRI and classified as a complete response, partial response, stable disease, or progressive disease (PD) using modified Response Evaluation Criteria in Solid Tumors.16 Disease control rate (DCR) was defined as the ratio of patients presenting a complete response, partial response, or stable disease at first radiological investigation. In 55 patients (5.8%), an assessment of tumor response by radiological imaging was not available.
Statistical analysis
Continuous variables are expressed as medians with the interquartile range. Categorical variables are given as relative and absolute frequencies. The Shapiro-Wilk test was used to test for a normal distribution of continuous variables. As there was no normal distribution of the patients, nonparametric tests were used to compare continuous variables between 2 groups. We applied χ2 for analysis of categorial variables. Survival analyses were performed using Kaplan-Meier curves and log-rank tests to determine survival differences. Logistic regression models were used to evaluate predictive factors of PES and severe PES. Parameters with a p-value < 0.05 (p-in) and p-out value of 0.1 were entered into the multivariable, bidirectional stepwise regression model starting with an empty model. Predictive factors for DCR were analyzed using logistic regression models. In the multivariable model, parameters with a p-value < 0.1 in the univariable models entered the multivariable model. Overall survival was analyzed by calculating the cumulative incidence function considering liver transplantation as a competing event. Prognostic factors were analyzed using multivariable Fine and Gray competing risk regression models. p-values < 0.05 were considered significant. Statistical analyses were performed in GraphPad Prism (version 9, GraphPad Software, San Diego, CA), R version 4.1.2, and STATA (Version 18.0, Stata Corp Lp., TX).
RESULTS
Patient characteristics
A total of 954 patients with HCC treatment by TACE were included in this study. Baseline characteristics of all patients and stratified according to the development of PES and its severity are shown in Table 2 and Supplemental Table S1, http://links.lww.com/HC9/A506. The majority of included patients received DEB-TACE (n = 538, 56.4%), 416 (43.6%) patients were treated by cTACE. In 782 patients (81.9%) TACE was the first treatment for HCC. However, 13.9% (n = 133) of patients previously underwent liver resection, 3.2% (n = 31) were previously treated with radiofrequency ablation, and 2.6% (n = 25) were previously treated with systemic therapy. Three patients (0.3%) had previous liver transplantation. The etiology of the underlying liver disease comprised all common risk factors, including alcohol-associated steatohepatitis (n = 404, 42.3%), viral hepatitis (n = 276, 28.8%), and NASH (n = 73, 7.7%); 311 patients (32.6%) were treated at early stage (BCLC A), whereas 514 patients (53.9%) were allocated to the BCLC B group. One hundred patients (10.5%) had advanced HCC (BCLC C). The majority of patients (n = 736, 77.1%) presented with multifocal disease.
TABLE 2.
Baseline characteristics of patients
| All (n = 954), n (%) | PES (n = 616), n (%) | No PES (n = 338), n (%) | p (PES vs. no PES), n (%) | |
|---|---|---|---|---|
| Age, y | 67.00 (60.00–74.00) | 68.00 (60.00–75.00) | 66.00 (58.00–74.00) | 0.01a |
| Sex | ||||
| Male | 834 (87.4) | 526 (85.3) | 308 (91.7) | 0.01b |
| Female | 120 (12.6) | 90 (14.6) | 30 (8.9) | — |
| Etiology of liver cirrhosis/HCC | ||||
| Alcohol-associated steatohepatitis | 404 (42.3) | 249 (40.4) | 155 (45.9) | 0.08b |
| Chronic HCV infection | 187 (19.6) | 114 (18.5) | 73 (21.6) | — |
| Chronic HBV infection | 88 (9.2) | 60 (9.7) | 28 (8.3) | — |
| NASH | 73 (7.7) | 47 (7.6) | 26 (7.7) | — |
| Other/unknown | 202 (21.1) | 146 (23.7) | 56 (16.6) | — |
| Viral status | ||||
| HBV: viral suppressionc | 60 (68.2) | 41 (68.3) | 19 (5.6) | 0.46b |
| HBV: no viral suppression | 10 (11.4) | 8 (13.3) | 2 (5.9) | — |
| HCV: SVRc | 45 (24.0) | 23 (3.7) | 22 (6.5) | 0.046b |
| HCV: no SVR | 104 (55.6) | 71 (11.5) | 33 (9.8) | — |
| BCLC stage | ||||
| 0 | 29 (3.0) | 17 (2.8) | 12 (3.6) | 0.0003b |
| A | 311 (32.6) | 172 (27.9) | 139 (41.1) | — |
| B | 514 (53.9) | 358 (58.1) | 156 (46.2) | — |
| C | 100 (10.5) | 69 (11.2) | 31 (9.2) | — |
| Tumor characteristics | ||||
| Solitary HCC | 218 (22.9) | 145 (23.6) | 73 (21.6) | 0.49b |
| Multifocal HCC | 736 (77.1) | 471 (76.5) | 265 (78.4) | — |
| No. nodules | 2.00 (1.00–4.00) | 2.00 (1.00–4.00) | 2.00 (1.00–4.00) | 0.16a |
| Max. tumor diameter (cm)d | 4.00 (3.00–06.00) | 4.00 (3.00–6.00) | 3.00 (2.55–5.00) | <0.0001a |
| Macrovascular invasion | 78 (8.2) | 52 (8.4) | 26 (7.7) | 0.23b |
| Extrahepatic metastases | 35 (3.7) | 22 (3.6) | 13 (3.8) | — |
| Tumorous PVT | 83 (8.7) | 54 (8.8) | 20 (5.9) | — |
| Nontumorous PVT | 95 (10.0) | 55 (8.9) | 40 (11.8) | — |
| Stage of liver cirrhosis | ||||
| Unknown | 42 (4.4) | 21 (3.4) | 21 (6.2) | 0.0006b |
| No cirrhosis | 64 (6.7) | 51 (8.3) | 13 (3.8) | — |
| CHILD A | 540 (56.6) | 364 (59.1) | 176 (52.1) | — |
| CHILD B | 308 (32.3) | 180 (29.2) | 128 (37.9) | — |
| CHILD C | 0 | 0 | 0 | — |
| Albumin/bilirubin Score (ALBI) | −2.147 (−2.59 to −1.585) | −2.182 (−2.65 to −1.685) | −2.049 (−2.541 to −1.470) | 0.009a |
| ALBI1 | 198 (20.8) | 138 (22.4) | 59 (17.5) | 0.02a |
| ALBI2 | 309 (32.4) | 301 (48.9) | 156 (46.2) | — |
| ALBI3 | 342 (35.8) | 81 (13.1) | 64 (18.9) | — |
| Performance status | ||||
| Unknown | 21 (2.2) | 2 (0.3) | 1 (0.3) | 0.53b |
| ECOG 0 | 191 (20.0) | 132 (21.4) | 59 (17.5) | — |
| ECOG 1 | 569 (59.6) | 360 (58.4) | 209 (61.8) | — |
| ECOG 2 | 173 (18.1) | 122 (19.8) | 69 (20.4) | — |
| Previous therapy | ||||
| None | 782 (81.9) | 503 (81.7) | 272 (80.5) | 0.44b |
| Liver transplantation | 3 (0.3) | 2 (0.3) | 1 (0.3) | — |
| Radiofrequency ablation | 31 (3.2) | 17 (2.8) | 14 (4.1) | — |
| Surgical resection | 133 (13.9) | 79 (12.8) | 54 (16.0) | — |
| TACE | 1 (0.1) | 1 (0.2) | 0 | — |
| Stereotactic body radiotherapy | 2 (0.2) | 2 (0.3) | 0 (0.0) | — |
| Systemic therapy | 25 (2.6) | 19 (3.1) | 6 (1.8) | — |
| Drug delivery method | ||||
| Conventional TACE | 416 (43.6) | 231 (37.5) | 185 (54.7) | 0.0001b |
| DEB-TACE | 538 (56.4) | 385 (62.5) | 153 (45.3) | — |
| Chemotherapeutic agent | ||||
| Mitomycin C | 421 (44.1) | 243 (39.4) | 178 (52.7) | 0.0001b |
| Epi-/doxorubicin | 611 (64.0) | 443 (71.9) | 168 (49.7) | — |
Note: Values are given as n (%) or median (IQR).
Mann-Whitney U test.
Chi-squared test.
Viral suppression defined as HBV titer <20 IU/mL, SVR defined as nondetectable HCV DNA.
Refers to the diameter of the largest tumor nodule.
Abbreviations: ALBI, Albumin/bilirubin Score; BCLC, Barcelona Clinic Liver Cancer; DEB-TACE, drug-eluting bead transarterial chemoembolization; ECOG, Eastern Cooperative Oncology Group;PES, postembolization syndrome; SVR, sustained viral response.
PES was diagnosed in 616 (64.6%) patients. The majority had mild (n = 168, 17.6%) or moderate (n = 392, 41.1%) symptoms, with only 56 patients (5.9%) suffering from severe PES. Radiological assessment of the tumor response by modified Response Evaluation Criteria in Solid Tumors16 after TACE indicated a DCR of 81.3%. Thus, partial response or complete response was observed in 478 patients (50.1%), stable disease in 298 patients (31.2%), and PD in 123 patients (12.9%). Median overall survival was 16.0 months (95% CI = 13.9–18.0]. The 1-year and 3-year survival were 49.4% and 13.3%, respectively. Further outcome variables are shown in Table 3.
TABLE 3.
Clinical outcome variables
| n = 954 patients, n (%) | |
|---|---|
| Development of PES | 616 (64.6) |
| PES grade | |
| Mild | 168 (17.6) |
| Moderate | 392 (41.1) |
| Severe | 56 (5.9) |
| mRECIST at 30–90 d | |
| Partial or complete response | 478 (50.1) |
| Stable disease | 298 (31.2) |
| Progressive disease | 123 (12.9) |
| Unknown | 55 (5.8) |
| Liver transplantation | 111 (11.6) |
| Median overall survival (mo) | 16.0 (95% CI, 13.9–18.0) |
Abbreviation: mRECIST, Modified Response Evaluation Criteria in Solid; PES, postembolization syndrome.
Tumor size, degree of cirrhosis, and DEB-TACE are predictors of PES occurrence
Next, we evaluated clinical risk factors for the development of PES. As shown in Figure 1A, we observed a strong correlation between tumor size and PES development (4.0 vs. 3.0 cm; p < 0.0001, Figure 1A). Moreover, despite similar tumor characteristics in patients treated with DEB-TACE or cTACE (Supplemental Table S2, http://links.lww.com/HC9/A506), patients who were treated with DEB-TACE more frequently developed PES compared to patients treated with cTACE (71.6% vs. 55.5%; p < 0.0001, Figure 1B). Importantly, DEB-TACE did not affect the frequency of severe PES in these patients (Supplemental Table S3, http://links.lww.com/HC9/A506). Several laboratory parameters were associated with PES post-TACE; preinterventional levels of INR (1.163 vs. 1.203, p = 0.003) and bilirubin (1.314 vs. 1.521, p = 0.0002) were significantly lower in patients experiencing PES following TACE (Figure 1C, D). In contrast, albumin (35.02 vs. 33.92 g/dL, p = 0.049) and platelet levels (159.2 vs. 144.1 ×103/µL, p = 0.007) were significantly higher in patients with PES (Figure 1E, F). Interestingly, in patients with chronic HCV infection, antiviral therapy was associated with a significantly decreased risk of PES (51.1% vs. 68.2%, p = 0.046, Table 1). In patients with HBV-infection, no statistical difference was found between viral suppression compared to no antiviral therapy (Table 1). Of note, PES occurred significantly more frequently in patients without cirrhosis than patients with cirrhosis (79.7% vs. 64.1%, p = 0.01, Figure 1G). In addition, patients with Child-Pugh stage A cirrhosis developed PES more frequently compared to patients with CHILD B cirrhosis, corroborating not only presence but also severity of cirrhosis as a protective factor for PES after TACE (67.4% vs. 58.4%; p = 0.009, Figure 1H). However, liver cirrhosis did not impact PES severity (Supplemental Table S4, http://links.lww.com/HC9/A506). Finally, multivariable logistic regression validated the presence of cirrhosis (OR = 0.48, 95% CI = 0.27–0.84, p = 0.01), tumor diameter (OR = 1.21, 95% CI = 1.13–1.28, p < 0.001), and DEB-TACE (OR = 1.99, 95% CI = 1.53–2.64, p < 0.001) as independent predictive factors of PES development (Table 4). Large tumor diameter was the only parameter independent risk factor for occurrence of severe PES (OR = 1.30, 95% CI = 1.19–1.42, p < 0.001, Supplemental Table S5, http://links.lww.com/HC9/A506).
FIGURE 1.
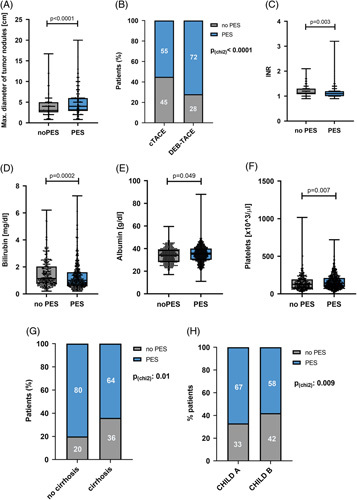
Predictive factors for occurrence of PES in patients with HCC after TACE. (A) Maximum diameter of tumor nodules in patients with PES compared to patients without PES after TACE (p < 0.0001, U test). (B) Frequency of PES in patients with HCC receiving conventional or DEB-TACE (p < 0.0001, χ2 test). (C) INR (international normalized ratio) in patients with PES compared to patients without PES after TACE (p = 0.003, U test). (D) Bilirubin level (mg/dL) in patients with PES compared to patients without PES after TACE (p = 0.0002, U test). (E) Albumin level (g/dL) in patients with PES compared to patients without PES (p = 0.049, U test). (F) Platelets (<103/μL) in patients with PES compared to patients without PES (p = 0.007, U test). (G) Frequency of PES in patients with or without cirrhosis (p = 0.01, χ2 test). (H) Frequency of PES in patients with CHILD A or CHILD B cirrhosis (p = 0.009, χ2 test). Box plots show individual data points, median, interquartile range, minimum, and maximum. Abbreviations: DEB-TACE, drug-eluting bead transarterial chemoembolization; PES, postembolization syndrome.
TABLE 4.
Prediction of PES in the overall cohort
| Univariable model | Multivariable model | |||
|---|---|---|---|---|
| Parameter | OR (95% CI) | p | OR (95% CI) | p |
| Age | 1.02 (1.00–1.04) | 0.004 | 0.41 (0.19–0.88) | 0.022 |
| Child score | 0.87 (0.76–0.96) | 0.009 | 0.89 (0.79–0.99) | 0.048 |
| Cirrhosis | 0.48 (0.27–0.84) | 0.011 | 0.89 (0.79–0.99) | 0.048 |
| Viral suppressiona | 0.87 (0.57–1.83) | 0.504 | — | — |
| Multifocal vs. solitary | 0.88 (0.63–1.21) | 0.492 | — | — |
| Max. diameter (cm) | 1.21 (1.13–1.28) | <0.001 | 1.20 (1.12–1.30) | <0.001 |
| Tumorous PVT | 1.04 (0.65–1.67) | 0.873 | — | — |
| Nontumorous PVT | 0.72 (0.48–1.09) | 0.119 | — | — |
| DEB-TACE | 1.99 (1.53–2.64) | <0.001 | 1.71 (1.24–2.35) | <0.001 |
Viral suppression was defined as HBV titer ≤20 IU/mL or nondetectable HCV DNA.
Abbreviation: DEB-TACE, drug-eluting bead transarterial chemoembolization.
Occurrence of severe PES is associated with reduced treatment response and poor prognosis
We next aimed to evaluate the role of PES development for clinical outcome after TACE. Patients with PES after TACE had significantly more frequent PD in radiological assessments after TACE (15.7% vs. 10.3%, p = 0.02, Figure 2A). Furthermore, the DCR correlated with PES grading and was lowest in patients with severe PES post-TACE (severe PES vs. no PES: 80% vs. 90%, p = 0.04, Figure 2B). Multivariable logistic regression validated PES (OR = 0.33, 95% CI = 0.16–0.69, p = 0.003), in addition to tumor size (OR = 0.89, 95% CI = 0.82–0.93, p = 0.02) as an independent negative predictor of DCR after TACE (Table 5). Finally, a multivariable competing risk model revealed severe PES (subdistribution HR = 1.53, 95% CI = 0.99–2.36, p = 0.04) in addition to CHILD score (HR = 1.15, 95% CI = 1.07–1.24, p < 0.001), tumor diameter (HR = 1.07, 95% CI = 1.04–1.11, p < 0.001), multifocal HCC (HR = 1.45, 95% CI = 1.17–1.79, p = 0.001), and tumorous PVT (HR = 2.25, 95% CI = 1.80–2.82, p < 0.001) as an independent predictor of poor overall survival in patients with HCC after TACE (Table 6).
FIGURE 2.
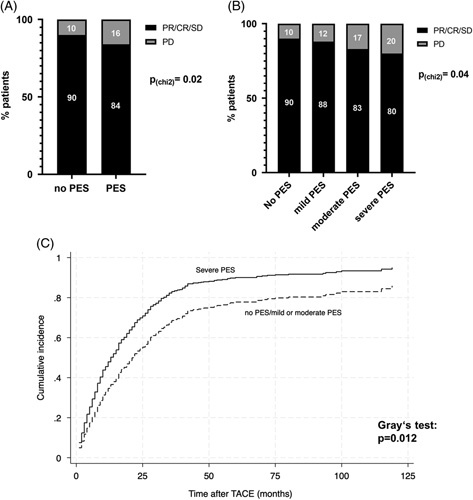
PES in HCC patients after TACE is associated with poor prognosis. (A) Frequency of disease control and PD in patients with or without PES after TACE (p = 0.02, χ2 test). (B) Frequency of disease control and PD in patients with mild, moderate, severe, or no PES after TACE (p = 0.04, χ2 test). (C) The cumulative incidence of death in patients with HCC and severe PES compared to patients without, mild or moderate PES. Liver transplantation was considered as competing risk. (p = 0.012; Gray test). Abbreviations: CR, complete response; PD, progressive disease; PES, postembolization syndrome; PR, partial response; SD, stable disease; TACE, transarterial chemoembolization.
TABLE 5.
Clinical parameters with impact on disease control rate assessed using univariable and multivariable logistic regression
| Univariable model | Multivariable model | |||
|---|---|---|---|---|
| Parameter | OR (95% CI) | p | OR (95% CI) | p |
| Cirrhosis | 1.23 (0.48–3.12) | 0.662 | — | — |
| Child score | 0.94 (0.72–1.22) | 0.623 | — | — |
| Multifocal vs. solitary | 1.55 (0.86–2.81) | 0.145 | — | — |
| Max. diameter (cm) | 0.89 (0.82–0.93) | 0.020 | 0.92 (0.84–1.01) | 0.095 |
| Tumorous PVT | 1.23 (0.43–3.48) | 0.699 | — | — |
| Nontumorous PVT | 1.58 (0.56–4.49) | 0.390 | — | — |
| DEB-TACE | 0.73 (0.42–1.28) | 0.266 | — | — |
| PES | 0.33 (0.16–0.69) | 0.003 | 0.30 (0.13–0.74) | 0.008 |
Abbreviations: DEB-TACE, drug-eluting bead transarterial chemoembolization; PES, postembolization syndrome.
TABLE 6.
Multivariable competing risk model for prediction of overall survival including liver transplantation as a competing risk
| Parameter | SHR (95% CI) | p |
|---|---|---|
| Cirrhosis | 0.92 (0.67–1.26) | 0.606 |
| Child score | 1.15 (1.07–1.24) | <0.001 |
| Multifocal vs. solitary | 1.45 (1.17–1.79) | 0.001 |
| Max. diameter (cm) | 1.07 (1.04–1.11 | <0.001 |
| Tumorous PVT | 2.25 (1.80–2.82) | <0.001 |
| No tumorous PVT | 0.77 (0.52–1.12) | 0.178 |
| DEB-TACE | 1.17 (0.97–1.40) | 0.098 |
| Severe PES | 1.53 (0.99–2.36) | 0.041 |
Abbreviations: DEB-TACE, drug-eluting bead transarterial chemoembolization; PES, postembolization syndrome; SHR, subdistribution HR.
Predictive factors for development of PES and its prognostic impact can be verified in a subgroup of patients with BCLC 0-B and no or CHILD A liver cirrhosis
The overall patient cohort in this study reflects a real-world cohort of patients with HCC treated by TACE at various tumor stages (BCLC 0-C, Table 1). However, current European guidelines recommend TACE preferentially in patients with intermediate stage HCC and preserved liver function (BCLC B). In order to validate predictive factors for PES and its prognostic impact in ideal candidates for TACE, we performed further regression analyses in a subgroup of patients with BCLC stages 0-B and no or CHILD A liver cirrhosis (n = 224 patients, 23.5% of the overall cohort, Supplemental Tables S6–S9, http://links.lww.com/HC9/A506). As shown in Supplemental Table S6, http://links.lww.com/HC9/A506, we could validate tumor size and DEB-TACE as independent risk factors for development of PES (OR: 1.24, 95% CI = 1.05–1.47, p = 0.02 and OR = 2.13, 95% CI = 1.19–3.82, p = 0.01, Supplemental Table S6, http://links.lww.com/HC9/A506). Cirrhosis, on the other hand, reappeared as a protective factor in terms of PES development, similar to the observation in the overall cohort (OR: 0.23, 95% CI = 0.06–0.82, p = 0.03, Supplemental Table S6, Supplemental Figure S1, http://links.lww.com/HC9/A506). Further corroborating the prognostic impact observed in our overall cohort, severe PES showed association with a worse overall survival in the multivariable competing risk model (subdistribution HR = 2.31, 95% CI = 0.99–5.34, p = 0.05, Supplemental Table S9, http://links.lww.com/HC9/A506).
DISCUSSION
PES represents a common complication following TACE in patients with HCC.17 Despite its high clinical impact as the strongest predictor of protracted recovery following TACE,17 the definition of PES is inconsistent, leading to highly variable incidences in different studies.10,11,17 In line with this, the predictive factors of PES development and its prognostic impact are controversial. Although tumor burden and dosage of chemoembolic agents have been consistently identified as risk factors of PES occurrence,10,17,18 heterogeneous reports exist on the predictive value of laboratory parameters of liver function and mode of TACE.13,19–21 Finally, a few small studies suggest a prognostic impact of PES development on overall survival following TACE,11,12 but conclusive and clinical translatable evidence is lacking.
These heterogeneous results are mainly due to the inconsistent definition of PES and investigations of small study cohorts. By stratifying the syndrome complex into different grades, we aimed to elucidate the predictive and prognostic factors of PES severity in patients undergoing TACE.
To the best of our knowledge, our cohort of 954 patients comprises the largest study cohort for the evaluation of predictive factors of PES in patients with HCC following TACE. In addition to previously described positive correlations between tumor burden and PES development,10,17,18 our study suggests DEB-TACE as an independent predictor of PES. Given that DEB-TACE was developed to decrease the systemic drug concentration compared to cTACE,22,23 these data are surprising but in line with recent observations in smaller study cohorts.13,19 Pathophysiologically, an increased risk of PES may be due to higher and sustained local drug concentrations.24,25 However, initial phase 2 studies suggested superior safety of DEB-TACE over cTACE.25 One reason for these contrasting results may be the characteristics of the study design. Thus, in the PRECISION V phase 2 trial, mostly patients with low tumor burden were included and treated by DEB-TACE with a doxorubicin or epirubicin dose of only up to 150 mg.25 In our study, on the other hand, tumor burden was higher (mean number of lesions in patients treated by DEB-TACE: 3.19 vs. 2.8 in PRECISION V) and the majority of patients had multiple tumor nodules (75.1% vs. 37.6% in PRECISION V). Moreover, as DEB-TACE only affects the frequency of PES in general, but not PES severity, a deviating definition of PES in the PRECISION V phase 2 trial may have limited the recognition of mild and moderate PES as complications.25 Importantly, in our study, age, tumor burden, grade of liver cirrhosis, and frequency of tumorous PVT were similar in patients undergoing DEB-TACE or cTACE, corroborating DEB-TACE as an independent predictor of PES in our multivariable regression model. Nevertheless, future prospective studies are needed for unbiased verification of DEB-TACE as a risk factor for PES.
Interestingly, our study identified the presence and degree of liver cirrhosis as a protective factor against PES. Rmilah et al20 recently reported similar findings in a cohort of patients with hepatic malignancies undergoing bland embolization. Pathophysiologically, necrosis of metabolically active tumor-adjacent liver tissue may induce stronger inflammation than cirrhotic tissue, which is characterized by enrichment of extracellular matrix and nonparenchymal cells.
Khalaf et al17 previously introduced PES grading based on the duration of hospitalization. As patients with TACE of HCC are regularly monitored in hospital wards in Germany for 1–3 days, and discharge is frequently influenced by patient preference, socioeconomic status, and reimbursement issues, we introduced a symptom-dependent severity score in this study. The resulting objective severity grades correlate with the varying definitions of PES in previous studies.9,11 The relevance of PES grading becomes apparent in our comprehensive analyses of the treatment response and prognosis. Thus, our study revealed, for the first time, an association of PES with the treatment response as patients with PES had a significantly increased risk of PD after TACE. This was further associated with a significantly impaired overall survival rate especially in patients with severe PES. Other independent prognostic factors in HCC patients treated with TACE identified tumor burden, PVT, and Child score as described.26,27
In line with meta-analyses on the safety and efficacy of cTACE and DEB-TACE in HCC patients,21,28,29 the presence of liver cirrhosis and DEB-TACE did not impact the risk of prognostically relevant severe PES. Collectively, these data have high clinical relevance and suggest routine clinical stratification of patient symptoms and enforced clinical monitoring of individuals with severe PES.
Our study has some limitations. We have defined PES severity according to presence and extent of the syndrome-defining clinical symptoms abdominal pain, fever, and nausea or vomiting since these symptoms guide clinical treatment. However, prognostic relevant association of severe PES with poor clinical outcome needs external validation. Next, the high number of patients allowed us to assess predictive and prognostic factors in multivariable regression models in order to consider relationships between multiple variables. However, due to the retrospective design of this study, bias of patient allocation to different TACE modalities cannot be excluded and could have impacted the prediction models. We also did not control for previous tumor therapies in our patient cohort. However, only the number of previous TACEs has been shown to affect the risk of PES occurrence,17 and our patients received TACE for the first time. In line, due to the retrospective character of our analyses and preventive treatment of patients with PES after first TACE in following TACE cycles, we were unable to evaluate the recurrence rate of PES. Future prospective studies should investigate predictors of repetitive severe PESs and its effect on tumor response and survival. In 55 of the 954 patients (5.8%), an assessment of tumor response by radiological imaging was not available, partly due to early death shortly after TACE. In other cases, patients did not keep follow-up appointments. To minimize bias due to loss to follow-up, these patients were excluded from the tumor response analysis, but not from the survival analysis. The overall survival of 16 months in our cohort is lower than expected based on recent survival data.30,31 As our cohort includes patients treated by TACE from 2005, these observations could be associated with improvements in the treatment modality and general medical care in recent years. Moreover, our cohort included a high proportion of patients with multilocular HCC, which is associated with poor prognosis.32 Finally, patients’ symptoms were only monitored for the duration of their hospital stay. Previous reports indicate persistence of symptoms for 1–2 weeks after interventions33; therefore, future studies should record the length and severity of patients’ symptoms after hospital discharge.
CONCLUSIONS
This dual-center study identified tumor burden, absence of liver cirrhosis, and DEB-TACE as strong predictive factors for the occurrence of PES. Moreover, our study revealed severe PES as one of the strongest independent risk factors for impaired tumor response and poor overall survival, indicating the necessity of categorical monitoring of patients’ symptoms after TACE.
Supplementary Material
Footnotes
Abbreviations: ALBI, Albumin-Bilirubin Score; BCLC, Barcelona Clinic Liver Cancer; cTACE, conventional transarterial chemoembolization; CR, complete response; DCR, disease control rate; DEB-TACE, drug-eluting bead transarterial chemoembolization; mRECIST, Modified Response Evaluation Criteria in Solid Tumors; PD, progressive disease; PES, postembolization syndrome; PR, partial response; SD, stable disease; SHR, subdistribution HR; TACE, transarterial chemoembolization.
Dominik Bettinger and Roman Kloeckner contributed equally.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepcommjournal.com.
Contributor Information
Natascha Roehlen, Email: natascha.roehlen@uniklinik-freiburg.de.
Fabian Stoehr, Email: fabian.stoehr@unimedizin-mainz.de.
Lukas Müller, Email: lukas.mueller@unimedizin-mainz.de.
Hendrik Luxenburger, Email: Hendrik.luxenburger@uniklinik-freiburg.de.
Simon J. Gairing, Email: sgairing@uni-mainz.de.
Marlene Reincke, Email: marlene.reincke@uniklinik-freiburg.de.
AUTHOR CONTRIBUTIONS
Roman Kloeckner and Dominik Bettinger designed the study. Fabian Stoehr, Lukas Müller, Michael Schultheiss, Marlene Reincke, Hendrik Luxenburger, and Floriona Berisha collected data. Natascha Roehlen, Floriona Berisha, and Dominik Bettinger analyzed the data. Natascha Roehlen, Dominik Bettinger, Floriona Berisha, and Roman Kloeckner interpreted the data. Natascha Roehlen drafted the manuscript. Roman Kloeckner and Dominik Bettinger edited the manuscript. Robert Thimme, Hendrik Luxenburger, Simon J. Gairing, Peter R. Galle, Arndt Weinmann, and Friedrich Foerster gave important intellectual input. All authors approved the final version of the article, including the authorship.
ACKNOWLEDGMENTS
The authors acknowledge support by the Open Access Publication Fund of the University of Freiburg.
FUNDING INFORMATION
We acknowledge support by the Open Access Publication Fund of the University of Freiburg. Natascha Roehlen and Michael Schultheiss are supported by the Berta-Ottenstein Programme, Faculty of Medicine, University of Freiburg. Hendrik Luxenburger is supported by the IMM-PACT-Programme for Clinician Scientists, Department of Medicine II, Medical Centre – University of Freiburg and Faculty of Medicine, University of Freiburg, funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, 413517907). Fabian Stoehr, Lukas Müller, and Simon J. Gairing are supported by the Clinician Scientist Fellowship “Else Kröner Research College: 2018_Kolleg.05.”
CONFLICTS OF INTEREST
Roman Kloeckner has received consultancy fees from Boston Scientific, Bristol-Myers Squibb, Guerbet, Roche, and SIRTEX, and lecture fees from BTG, EISAI, Guerbet, Ipsen, Roche, Siemens, SIRTEX, and MSD Sharp & Dohme. None are related to this work. Arndt Weinmann has received consultancy fees from Bayer, BMS, Sanofi, Roche, Astra Zeneca, Servier and lecture fees from Eisai, Ipsen, and MSD. None are related to this work. Dominik Bettinger has received consultancy fees from Boston Scientific and lecture fees from W.L. Gore & Associates and the Falk Foundation. The remaining authors have no conflicts to report.
REFERENCES
- 1. Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 3. Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kloeckner R, Galle PR, Bruix J. Local and regional therapies for hepatocellular carcinoma. Hepatology. 2021;73(suppl 1):137–49. [DOI] [PubMed] [Google Scholar]
- 5. Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: Modalities, indication, and patient selection. J Hepatol. 2015;62:1187–95. [DOI] [PubMed] [Google Scholar]
- 6. Miyayama S, Matsui O. Superselective conventional transarterial chemoembolization for hepatocellular carcinoma: Rationale, technique, and outcome. J Vasc Interv Radiol. 2016;27:1269–78. [DOI] [PubMed] [Google Scholar]
- 7. Prajapati HJ, Rafi S, El-Rayes BF, Kauh JS, Kooby DA, Kim HS. Safety and feasibility of same-day discharge of patients with unresectable hepatocellular carcinoma treated with doxorubicin drug-eluting bead transcatheter chemoembolization. J Vasc Interv Radiol. 2012;23:1286–93.e1. [DOI] [PubMed] [Google Scholar]
- 8. de Baere T, Ronot M, Chung JW, Golfieri R, Kloeckner R, Park J-W, et al. Initiative on superselective conventional transarterial chemoembolization results (INSPIRE). Cardiovasc Intervent Radiol. 2022;45:1430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blackburn H, West S. Management of postembolization syndrome following hepatic transarterial chemoembolization for primary or metastatic liver cancer. Cancer Nurs. 2016;39:E1–18. [DOI] [PubMed] [Google Scholar]
- 10. Lima M, Dutra S, Gomes FV, Bilhim T, Coimbra É. Risk factors for the development of postembolization syndrome after transarterial chemoembolization for hepatocellular carcinoma treatment. Acta Med Port. 2018;31:22–9. [DOI] [PubMed] [Google Scholar]
- 11. Mason MC, Massarweh NN, Salami A, Sultenfuss MA, Anaya DA. Post-embolization syndrome as an early predictor of overall survival after transarterial chemoembolization for hepatocellular carcinoma. HPB (Oxford). 2015;17:1137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shim JH, Park J-W, Choi J-I, Kim H-B, Lee WJ, Kim C-M. Does postembolization fever after chemoembolization have prognostic significance for survival in patients with unresectable hepatocellular carcinoma? J Vasc Interv Radiol. 2009;20:209–16. [DOI] [PubMed] [Google Scholar]
- 13. He J-J, Yin X-X, Wang T, Chen M-Y, Li X-L, Yang X-J, et al. Factors influencing postembolization syndrome in patients with hepatocellular carcinoma undergoing first transcatheter arterial chemoembolization. J Cancer Res Ther. 2021;17:777–83. [DOI] [PubMed] [Google Scholar]
- 14. Arslan M, Degirmencioglu S. Risk factors for postembolization syndrome after transcatheter arterial chemoembolization. Curr Med Imaging Rev. 2019;15:380–5. [DOI] [PubMed] [Google Scholar]
- 15. European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu, European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 16. Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72:288–306. [DOI] [PubMed] [Google Scholar]
- 17. Khalaf MH, Sundaram V, AbdelRazek Mohammed MA, Shah R, Khosla A, Jackson K, et al. A predictive odel for postembolization syndrome after transarterial hepatic chemoembolization of hepatocellular carcinoma. Radiology. 2019;290:254–61. [DOI] [PubMed] [Google Scholar]
- 18. Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12:321–6. [DOI] [PubMed] [Google Scholar]
- 19. Bian L-F, Zhao X-H, Gao B-L, Zhang S, Ge G-M, Zhan D-D, et al. Predictive model for acute abdominal pain after transarterial chemoembolization for liver cancer. World J Gastroenterol. 2020;26:4442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rmilah AA, Qrareya MN, Fleming C, Alkurashi AK, Nyberg S, Leise M, et al. Association of cirrhosis and other patient and procedural characteristics with postembolization syndrome after bland hepatic artery embolization for hepatic malignancy. AJR Am J Roentgenol. 2022;218:1030–9. [DOI] [PubMed] [Google Scholar]
- 21. Zou JH, Zhang L, Ren ZG, Ye SL. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: A meta-analysis. J Dig Dis. 2016;17:510–7. [DOI] [PubMed] [Google Scholar]
- 22. Sottani C, Poggi G, Quaretti P, Regazzi M, Montagna B, Quaquarini E, et al. Serum pharmacokinetics in patients treated with transarterial chemoembolization (TACE) using two types of epirubicin-loaded microspheres. Anticancer Res. 2012;32:1769–74. [PubMed] [Google Scholar]
- 23. Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–81. [DOI] [PubMed] [Google Scholar]
- 24. Song MJ, Chun HJ, Song DS, Kim HY, Yoo SH, Park C-H, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012;57:1244–50. [DOI] [PubMed] [Google Scholar]
- 25. Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: Results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mähringer-Kunz A, Steinle V, Düber C, Weinmann A, Koch S, Schmidtmann I, et al. Extent of portal vein tumour thrombosis in patients with hepatocellular carcinoma: The more, the worse? Liver Int. 2019;39:324–331. [DOI] [PubMed] [Google Scholar]
- 27. Müller L, Hahn F, Mähringer-Kunz A, Stoehr F, Gairing SJ, Foerster F, et al. Refining prognosis in chemoembolization for hepatocellular carcinoma: Immunonutrition and liver function. Cancers (Basel). 2021;13:3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiang H, Xiong B, Li H, Zhao C, Zhang Z, Ma C, et al. Comparison of liver function and safety in hepatocellular cancer patients treated with DEB-TACE and cTACE: A multi-center, retrospective cohort study. Transl Cancer Res. 2019;8:1950–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prateepchaiboon T, Chang A, Pungpipattrakul N, Akarapatima K, Rattanasupar A, Songjamrat A, et al. Factors affecting prognosis in hepatocellular carcinoma patients post-transarterial chemoembolization. Indian J Gastroenterol. 2022;41:352–61. [DOI] [PubMed] [Google Scholar]
- 30. Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330–5. [DOI] [PubMed] [Google Scholar]
- 31. Nam HC, Jang B, Song MJ. Transarterial chemoembolization with drug-eluting beads in hepatocellular carcinoma. World J Gastroenterol. 2016;22:8853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [DOI] [PubMed] [Google Scholar]
- 33. Yinglu F, Changquan L, Xiaofeng Z, Bai L, Dezeng Z, Zhe C. A new way: Alleviating postembolization syndrome following transcatheter arterial chemoembolization. J Altern Complement Med. 2009;15:175–181. [DOI] [PubMed] [Google Scholar]


