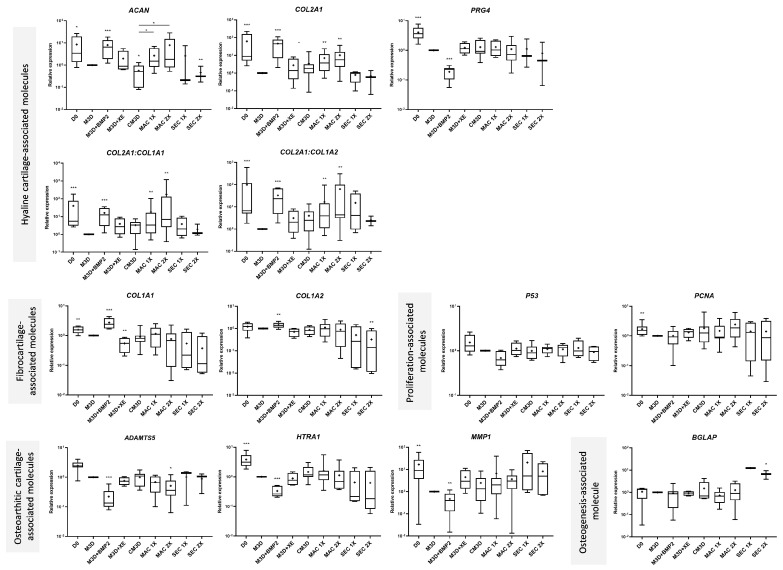
Figure 3.
Purification method changes the effect of exosomes from BM-MSCs on the eAC gene expression of cartilage- and OA-associated markers. CMs from BM-MSCs (P3) were harvested and exosomes were isolated using either the MAC or the SEC method. Unconditioned medium (M3D) was aliquoted, supplemented with fresh exosomes, and stored at −80 °C. Controls were included by supplementing M3D with BMP2 (50 ng/mL) or Qiagen elution buffer (XE) and CM3D corresponds to BM-MSC culture medium conditioned for 24 h. The 1X and 2X concentrations refer to protein concentrations of exosome solution related to 1 mL of CM3D. eACs (P2) were seeded in collagen sponges at 800,000 cells/sponge and then cultured under hypoxic atmosphere with the different media for 14 days. Then, sponges were harvested, washed twice with PBS, and stored at −80 °C. Total RNA was collected from these cultures and RT-qPCRs were run to assess gene expression. The expression of target genes was normalized using either the reference genes β-ACTIN and PPIA. The D0 condition corresponds to eACs cultured in monolayer until P2. Experiments were reproduced with different strains of eACs and BM-MSCs (n = 8; n = 4 for Izon conditions). Values are represented as box plots (median, quartiles, extreme values, “+” corresponds to the mean) and tested using the Mann–Whitney test, * p < 0.05, ** p < 0.01, *** p < 0.005 significantly different from the M3D condition. BM-MSCs, bone marrow-mesenchymal stromal cells; BMP2, bone morphogenetic protein 2; CM, conditioned medium; eACs, equine articular chondrocytes; M3D, control medium with 2% FBS; P2, passage 2; P3, passage 3; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; XE, Qiagen elution buffer.