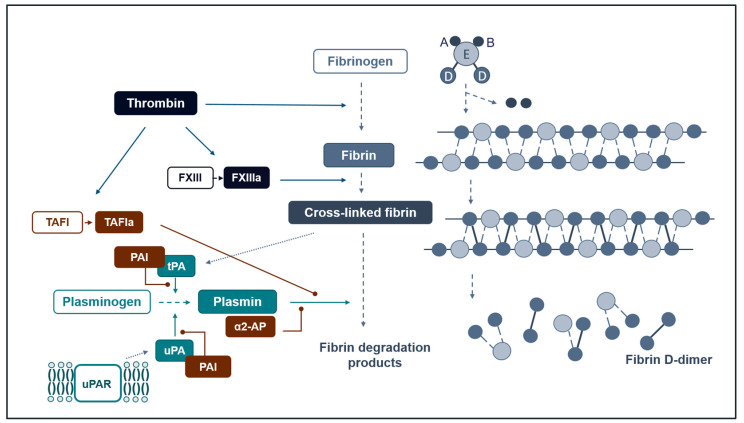
Figure 1.
Schematic overview of activators and regulators of fibrinolysis. As a result of coagulation activation, fibrinogen A and B domains are cleaved from fibrinogen by thrombin, giving rise to fibrin monomers, which subsequently polymerise to form the fibrin clot. The clot is then stabilised by covalent crosslinking of D-domains induced by coagulation factor (F) XIIIa. Plasmin is the main enzyme responsible for fibrin clot degradation (fibrinolysis) and is activated by tissue-type plasminogen activator (tPA) in the presence of fibrin, or by urokinase-type plasminogen activator (uPA) in the presence of its cellular receptor (uPAR). Important regulators of fibrinolysis are plasminogen activator inhibitors (PAI)-1 and -2, which inhibit tPA and uPA activity; α2-antiplasmin binds and inhibits plasmin directly, and thrombin-activatable fibrinolysis inhibitor (TAFI) cleaves lysine residues from the fibrin clot and thereby impedes plasminogen binding to fibrin. (a) denotes activated enzyme.