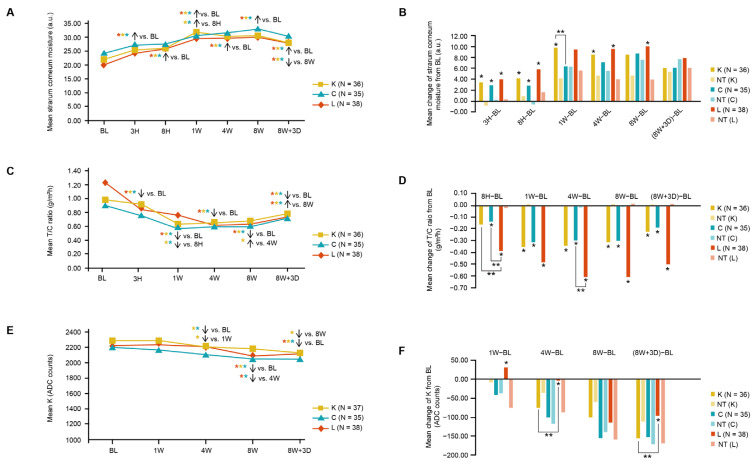
Figure 1.
Clinical efficacy parameters for the three investigated moisturizer groups and untreated areas across the 8-week randomized, controlled, and triple-blind clinical trial. Comparison of skin stratum corneum moisture content (measured using a Corneometer®) between the (A) time points of moisturizer groups and (B) moisturizers/NT; comparison of skin barrier function (T/C) between the (C) time points of moisturizer groups and (D) moisturizers/NT; comparison of skin translucency (K value; a lower K value indicates increased skin translucency) between the (E) time points of moisturizer groups and (F) moisturizers/NT. Abbreviations: 8 H, 8 h; 1 W, 1 week; 4 W, 4 weeks; 8 W, 8 weeks; 8 W + 3 D, 8 weeks + 3 days (3 days after treatment is stopped); ADC, analog-to-digital converter (light intensity count); a.u., arbitrary unit; BL, baseline; K, water gel with yeast extract; C, water gel; L, extra-dry emulsion; T/C, ratio of trans-epidermal water loss and surface moisture content measured by Corneometer® (which indicates the relative skin barrier function to skin water content). * p < 0.05. ** p < 0.01. p-values are derived using the t-test and ANOVA.