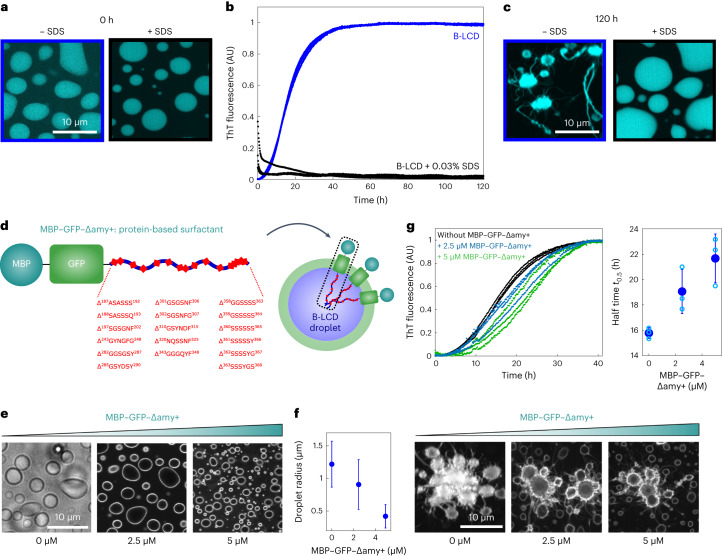
Fig. 5. Targeting the surface of condensates inhibits amyloid formation.
a–c, The addition of 0.03% SDS to a 30 µM B-LCD solution did not affect LLPS (a) but prevented the increase of ThT signal over time (b) as well as the formation of ThT-positive rims and star-shaped aggregates after 120 h, as shown by re-scan confocal microscopy images (c). Analysis of B-LCD droplets and starbursts in absence and presence of SDS was repeated three times yielding similar results. d, Design of a protein-based surfactant (MBP–GFP–Δamy+) consisting of soluble MBP, GFP and a B-LCD variant lacking all predicted steric zippers (Δamy+). e, Re-scan confocal fluorescence microscopy images showing accumulation of the protein-based surfactant molecules at the droplet surface. The fluorescent signal is originating from the GFP domain of the protein surfactant. Images were acquired at time 0. Experiments were independently repeated three times with similar results. f, Decrease of the average droplet size with increasing MBP–GFP–Δamy+ concentration. Error bars represent the standard deviation of sizes of 1,128 (0 µM MBP–GFP–Δamy+), 1,561 (2.5 µM MBP–GFP–Δamy+) and 1,020 (5 µM MBP–GFP–Δamy+) droplets from three independent samples. g, In addition to changing the size of the condensates, increasing concentrations of the protein-based surfactant delays amyloid formation inside condensates. Average half times t0.5 were extracted from the ThT profiles as a function of the MBP–GFP–Δamy+ protein surfactant. Black, 0 µM MBP–GFP–Δamy+; blue, 2.5 µM MBP–GFP–Δamy+; green, 5 µM MBP–GFP–Δamy+. Error bars represent the standard deviation of three independent samples. Re-scan confocal fluorescence microscopy images of samples after 40 h of incubation with increasing protein surfactant concentration.