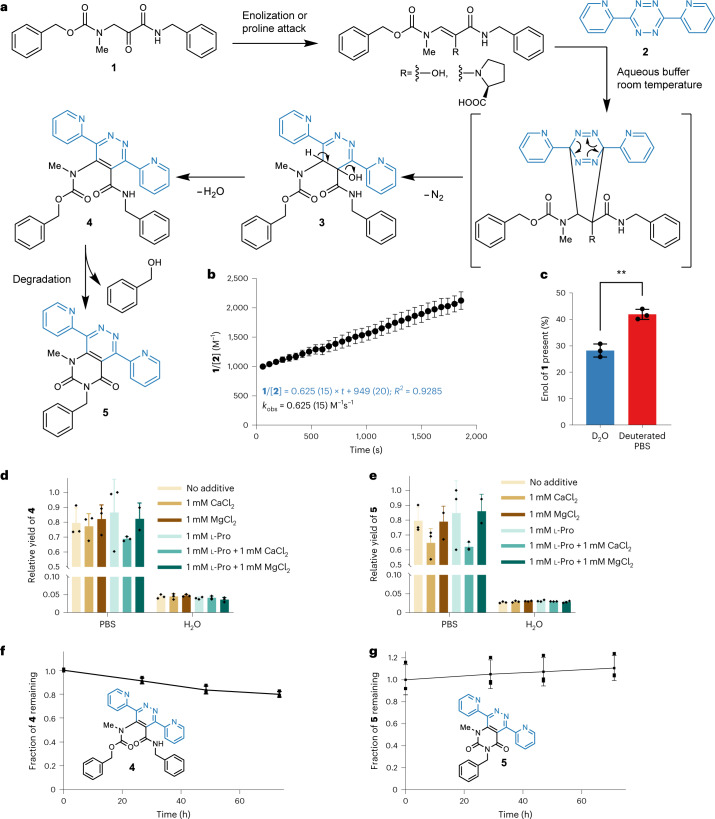
Fig. 2. Test reactions of the aminopyruvate model compound 1 to explore the feasibility of pyridazine formation in vitro.
a, Proposed reaction between 1 and 3,6-di-2-pyridyl-1,2,4,5-tetrazine 2 to yield intermediate 3, and pyridazine products 4 and 5. b, Reaction kinetics in PBS pH 7.4 measured by visible light spectrometry. The decrease in absorbance at 515 nm of tetrazine 2 was measured over time and fitted to a second-order reaction kinetic equation yielding an apparent rate constant of kobs = 0.625 (15) M−1 s−1 in 30% DMSO in H2O (n = 3 independent experiments). Points describe mean; error bars describe standard deviation. c, Enol form of 1 present in solution in D2O or deuterated PBS (28.2% (2.4%) in D2O, 41.9% (1.9%) in PBS, P = 0.0016 by a two-tailed t-test) measured by deuterium exchange and MS (n = 3 independent experiments). Bars describe mean; error bars describe standard deviation. d,e, Effect of medium, l-proline and divalent metal ion additives on the yields of pyridazine 4 (d) and 5 (e), estimated by MS product ion abundance and relative product formation (n = 3 independent experiments). Bars describe mean; error bars describe standard deviation. f,g, Stability of conjugate 4 (f) and 5 (g) over time in 50% acetonitrile in H2O, assessed by UV signal (280 nm). The line connects mean values; error bars represent standard deviations (n = 3 independent experiments).