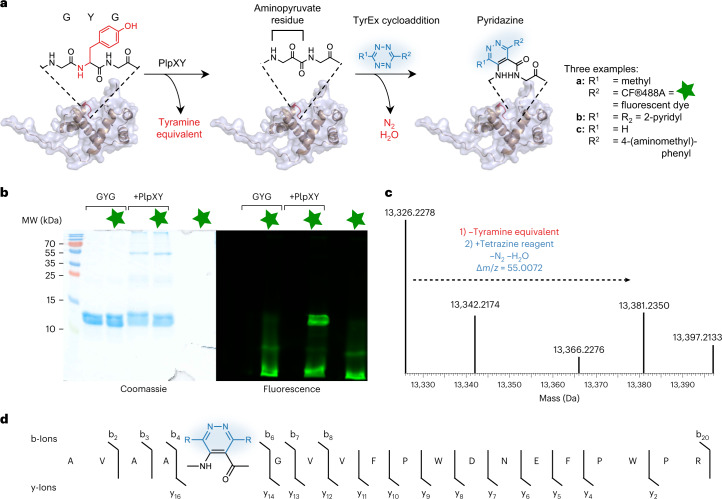
Fig. 3. In vitro peptide conjugation of His6-PlpA3-M5G with tetrazines.
a, Overall tyramine excision (TyrEx) strategy for labelling of His6-PlpA3-M5G: PlpXY expressed in the cell installs aminopyruvate site-specifically in the target biomolecule at a GYG-containing motif. Splicing results in a mass loss of −135.0757 Da (−C8H9NO). The post-translationally modified product reacts tetrazine derivatives to form the pyridazyl protein conjugates a–c. Structures are based on models calculated with AlphaFold47,48. b, Conjugation of His6-PlpA3-M5G with a fluorescent tetrazine probe analysed by SDS–PAGE. GYG: unmodified His6-PlpA3-M5G molecular weight (MW) 13.3 kDa. +PlpXY: His6-PlpA3-M5G was co-produced with PlpXY to yield the aminopyruvate-containing product (MW 13.2 kDa). The CF®488A fluorophore (6) was imaged by gel fluorescence using a 488 nm excitation and 532/528 nm emission filter. The experiment was performed once. c, Deconvoluted electrospray ionization–mass spectra (ESI–MS) showing ions for the substrate (His6-PlpA3-M5G, m/z calculated 13,326.2112, found 13,326.2278) and the cycloaddition product using tetrazine 2 (m/z calculated 13,381.2071, found 13,381.2350). d, Observed y- and b-ions for the ESI–MS/MS fragmentation of a trypsinized reaction product localizing the cycloaddition site (blue).