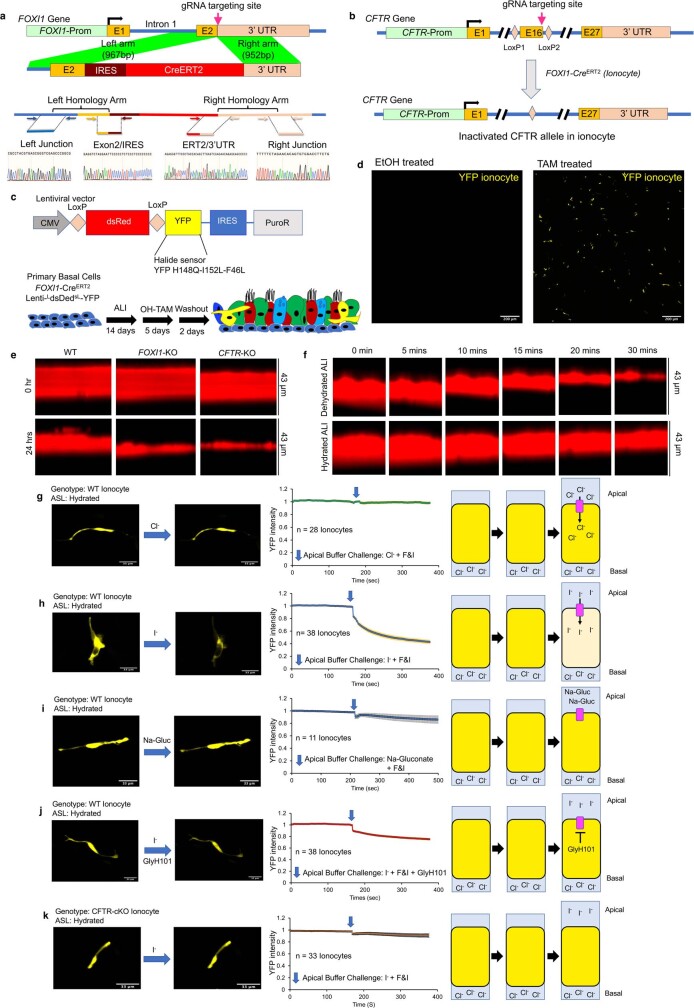
Extended Data Fig. 2. FOXI1-CreERT2 and CFTR conditional KO (CFTRL/L) ferret models demonstrate CFTR is required for ionocyte apical uptake of anions.
CRISPR homology-directed repair (HDR) was used to generate FOXI1-CreERT2 and CFTRL/L ferrets. a, Schematic of the strategy for generating transgenic ferrets with an IRES-CreERT2 insertion in the FOXI1 3’-UTR. b, Schematic of the floxed exon-16 in CFTRL/L ferrets and strategy for deletion of CFTR in FOXI1-CreERT2::CFTRL/L (CFTR-cKO) ferrets. c, Primary FOXI1-CreERT2 airway basal cells transduced with a lentivirus encoding LoxP-dsRED-stop-LoxP-YFP-H148Q/I152L cassette (herein called FOXI1-CreERT2::YFPH148Q/I152L) and differentiated at ALI, treated with hydroxy-tamoxifen (OH-Tam) and then used for functional studies of halide transport. d, Scattered YFP-positive ionocytes were observed in the pseudostratified airway epithelium of only OH-Tam treated differentiated FOXI1-CreERT2::YFPH148Q/I152L ALI airway cultures. Representative images from 3 independent ferret donors. e, Representative images of ASL height from differentiated WT, CFTR-KO, and FOXI1-KO ALI cultures challenged with 18 μl of Alexa-dye containing buffer (time zero) and following equilibration 24 hrs later. Representative images from n = 11 (WT), n = 10 (FOXI1-KO) and n = 9 (CFTR-KO) independent cultures. f, Dehydration experiment on WT ALI cultures monitoring the ASL height following apical perfusion of non-humidified 5% CO2 for the indicated times. 20 min of dehydration was chosen for basolateral halide sensor assays (Extended Data Fig. 3) since the ASL height approached that observed CFTR-KO and FOXI1-KO cultures. Representative images from 3 independent experiments. g–k, Representative images and traces of apical I– uptake in YFP halide sensor expression ionocytes of ALI cultures. g, No YFP quenching is observed in WT ionocytes after the addition of apical Cl– buffer (18 μl) with Forskolin/IBMX (F&I) to stimulate CFTR (negative control). Mean ± s.e.m.; n = 28 ionocytes. h, YFP quenching is observed in WT ionocytes following the addition of apical I– buffer with F&I. Mean ± s.e.m.; n = 38 ionocytes. i, YFP quenching, as shown in (h), is not observed following the addition of apical Na-Gluconate (Na-Gluc) buffer with F&I (negative control). Mean ± s.e.m.; n = 11 ionocytes. j, YFP quenching, as shown in (h), is reduced following the addition of apical I– buffer, F&I, and GlyH101 CFTR inhibitor (negative control). Mean ± s.e.m.; n = 38 ionocytes. k, YFP quenching is not observed in CFTR-cKO ionocytes (FOXI1-CreERT2::CFTRL/L) following application of apical I– buffer with F&I. Mean ± s.e.m.; n = 33 ionocytes.