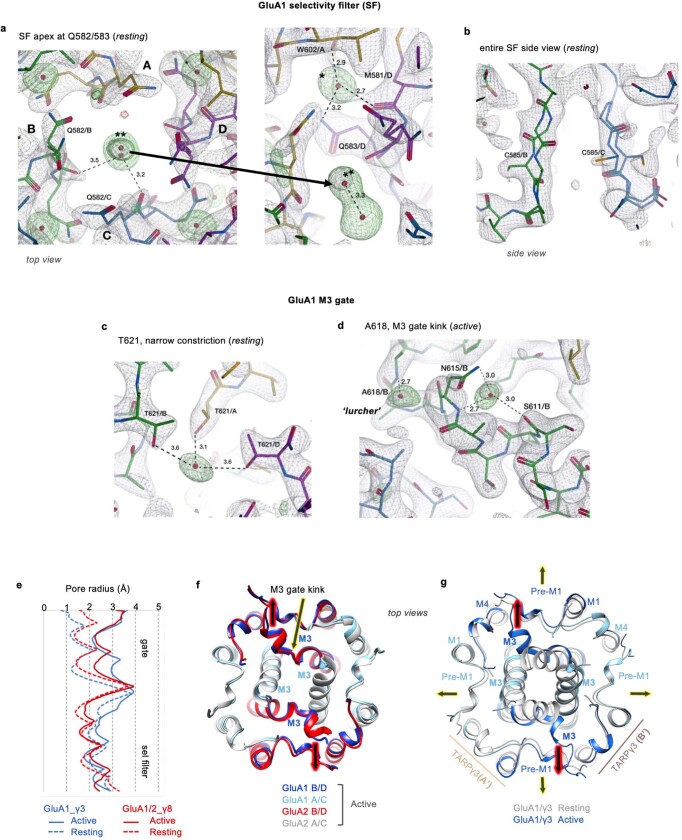
Extended Data Fig. 4. Features in the TMD of resting and open state GluA1/γ3.
a, Peaks corresponding to sodium ions observed at the selectivity filter entry in the resting state from top (left) and bottom (right) views. The putative Na+ ion is marked with two asterisks. A strong water molecule locates to the periphery of Q583, connecting it with M581 and W602 in a neighboring subunit (this water is denoted with one asterisk). b, side view onto the selectivity filter showing the mobile C585 modelled in double occupancy. c, A putative Na+ ion locates to the narrow M3 gate constriction and is coordinated by the four T621 side chains, one from each subunit. d, Open state-specific water molecules that may stabilize the M3 kink, a hallmark of the AMPAR open state. Hydrogen bonds indicated by stippled lines, with distances in Ångstrom shown. All Fo-Fc difference peaks (green mesh) are contoured at 5.5σ. e, Pore dimensions of resting state (stippled lines) and active states (full lines) for GluA1/γ3 (blue) and GluA1/2 (red; PDB: 7OCF). f, Top view of superposed active state GluA1/γ3 and GluA1/2_γ8/CNIH2 (PDB: 7OCF) models, showing close similarity and gating dominance of the B/D chains (black arrows, red glow). g, Top view of superposed GluA1/γ3 models at resting (grey) and open state (colored) shows the asymmetric gate dilation as in B, which is accompanied by widening of the preM1 helices in the active versus resting state.