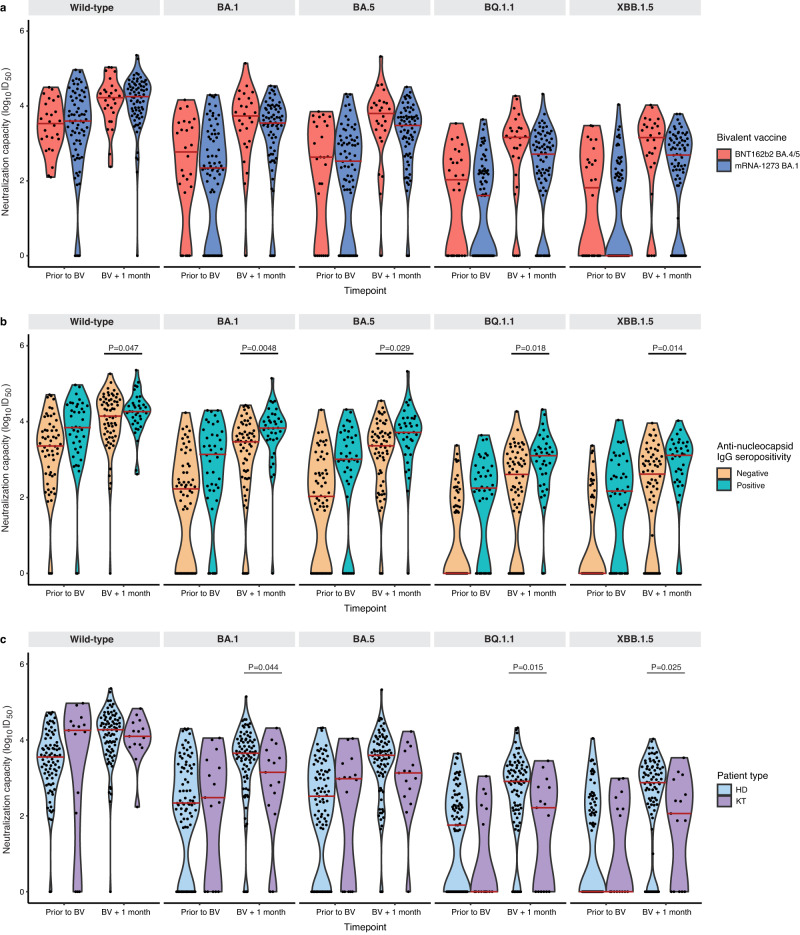
Fig. 2. Neutralizing antibodies against wild-type, BA.1, BA.5, BQ.1.1, and XBB.1.5 prior to and following bivalent vaccination.
a Stratified by vaccine type BNT162b2 BA.4/5 (n = 26) versus mRNA-1273 BA.1 (n = 72). Increases in neutralizing antibody levels were not significantly different by bivalent vaccine type for any subvariant after adjustment for anti-nucleocapsid positivity, hemodialysis versus kidney transplant recipient, and number of vaccine doses or (b) stratified by anti-nucleocapsid IgG seropositivity (n = 40) as a marker of prior COVID-19 infection versus seronegative (n = 58). Neutralizing antibody against Omicron subvariants were higher among those with anti-nucleocapsid seropositivity against wild-type (D614G) (P = 0.047), BA.1 (P = 0.0048), BA.5 (P = 0.029), BQ.1.1 (P = 0.018), and XBB.1.5 (P = 0.014) after adjusting for number of doses, vaccine type, patient type, and timepoint; or (c) stratified by patient type (hemodialysis n = 83) versus kidney transplant (n = 15). Solid red line indicates median level. Dots represent individual serum samples collected (n = 98 for each time point). Results were analysed using a linear mixed effects model, with a two-sided p-value. No adjustments were made for multiple comparisons.