Extract
The airway epithelium plays a central role in the initiation and development of chronic airway diseases [1]. It is the first line of defence against the external environment; therefore, numerous inflammatory and tissue remodelling pathways exist to protect against damage caused by constant exposure to inhaled stimuli (e.g. allergens, cigarette smoke or pathogens) [1, 2]. Dysregulation of these pathways alters the structure of the airway epithelium, making it an inadequate defence against further insult from inhaled stimuli. This results in a cycle of abnormal epithelial responses and further damage, which may contribute to irreversible airway changes and severe chronic airway disease, such as chronic obstructive pulmonary disease (COPD) [1, 2].
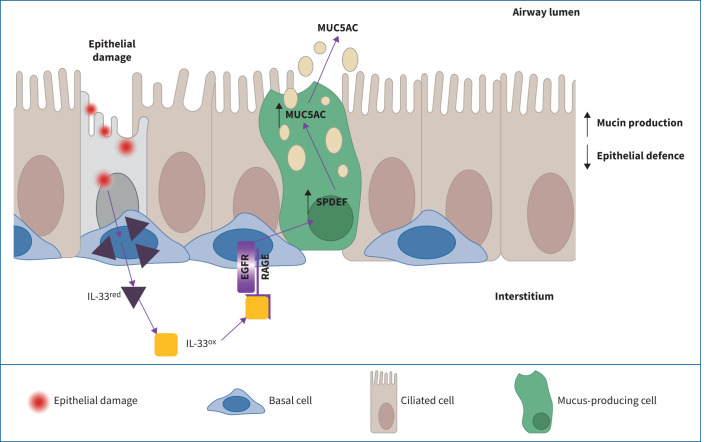
Graphical abstract
IL-33ox binds to receptor for advanced glycation end products (RAGE) to signal via epidermal growth factor receptor (EGFR). Activation of the IL-33ox–RAGE/EGFR pathway redirects epithelial cell fate, promoting a mucin hypersecretion phenotype at the expense of epithelial defence functions.
Abstract
Background
Epithelial damage, repair and remodelling are critical features of chronic airway diseases including chronic obstructive pulmonary disease (COPD). Interleukin (IL)-33 released from damaged airway epithelia causes inflammation via its receptor, serum stimulation-2 (ST2). Oxidation of IL-33 to a non-ST2-binding form (IL-33ox) is thought to limit its activity. We investigated whether IL-33ox has functional activities that are independent of ST2 in the airway epithelium.
Methods
In vitro epithelial damage assays and three-dimensional, air–liquid interface (ALI) cell culture models of healthy and COPD epithelia were used to elucidate the functional role of IL-33ox. Transcriptomic changes occurring in healthy ALI cultures treated with IL-33ox and COPD ALI cultures treated with an IL-33-neutralising antibody were assessed with bulk and single-cell RNA sequencing analysis.
Results
We demonstrate that IL-33ox forms a complex with receptor for advanced glycation end products (RAGE) and epidermal growth factor receptor (EGFR) expressed on airway epithelium. Activation of this alternative, ST2-independent pathway impaired epithelial wound closure and induced airway epithelial remodelling in vitro. IL-33ox increased the proportion of mucus-producing cells and reduced epithelial defence functions, mimicking pathogenic traits of COPD. Neutralisation of the IL-33ox pathway reversed these deleterious traits in COPD epithelia. Gene signatures defining the pathogenic effects of IL-33ox were enriched in airway epithelia from patients with severe COPD.
Conclusions
Our study reveals for the first time that IL-33, RAGE and EGFR act together in an ST2-independent pathway in the airway epithelium and govern abnormal epithelial remodelling and muco-obstructive features in COPD.
Tweetable abstract
An ST2-independent signalling complex of oxidised IL-33, receptor for advanced glycation end products (RAGE) and epidermal growth factor receptor (EGFR) governs epithelial remodelling and mucin hypersecretion in COPD https://bit.ly/3pxgaqx
Introduction
The airway epithelium plays a central role in the initiation and development of chronic airway diseases [1]. It is the first line of defence against the external environment; therefore, numerous inflammatory and tissue remodelling pathways exist to protect against damage caused by constant exposure to inhaled stimuli (e.g. allergens, cigarette smoke or pathogens) [1, 2]. Dysregulation of these pathways alters the structure of the airway epithelium, making it an inadequate defence against further insult from inhaled stimuli. This results in a cycle of abnormal epithelial responses and further damage, which may contribute to irreversible airway changes and severe chronic airway disease, such as chronic obstructive pulmonary disease (COPD) [1, 2].
COPD is the third leading cause of death worldwide and there is an urgent need to develop efficacious therapies [3]. An enhanced understanding of the COPD epithelial disease process will support rational drug design, which, in turn, will hopefully lead to improved patient outcomes. Airway epithelium from patients with COPD has been shown to exhibit hyperplasia of mucus-producing goblet cells and a reduction in the proportion of ciliated cells [4]. The key epithelial molecules underlying these changes remain under investigation; however, genetic analyses suggest that interleukin (IL)-33 drives the pathology of COPD and other chronic airway diseases, such as asthma. A rare loss-of-function mutation in IL-33 reduces the risk of COPD and asthma, whereas gain-of-function mutations are associated with an increased risk of both diseases [5, 6]. Increased IL-33 levels have been observed in serum, sputum and lung biopsy samples from patients with COPD and those with asthma [7–9]. Furthermore, serum IL-33 levels have been shown to correlate with disease severity and exacerbation history in COPD [8]. Despite these observations, the role of IL-33 in COPD remains poorly defined.
IL-33 is released from epithelial cells following damage induced by inhaled stimuli. It then binds to a cell surface receptor complex composed of serum stimulation-2 (ST2) (also known as IL-1 receptor-like 1) and IL-1 receptor accessory protein, which activates NF-ĸB inflammatory signalling pathways. Dysregulation of this pathway can lead to chronic airway inflammation [10]. Although the impact of IL-33 on immune cells is well established [11], there is a lack of clarity on the effect of IL-33 on epithelial cells. Other studies have reported that ST2, the only known IL-33 receptor to date, is expressed in the airway, thus providing the potential for IL-33 to elicit a direct effect on epithelial cells [12, 13].
Two redox forms of IL-33 have previously been described: a reduced form (IL-33red) and an oxidised form (IL-33ox) [14]. Oxidation of IL-33 results in the formation of disulfide bridges and a conformational change that prevents IL-33 from binding to ST2. IL-33 oxidation was initially proposed as a mechanism to limit IL-33 activity [14]. Here, we identify a previously undescribed ST2-independent IL-33 pathway, activated by IL-33ox, that has profound effects on the airway epithelium and implications for our understanding of COPD pathology.
Materials and methods
Detailed materials and methods are described in the supplementary material.
Results
ST2-independent effects of IL-33
We evaluated the role of IL-33 signalling in an epithelial scratch wound assay. High-affinity binding of IL-33 via an IL-33-neutralising monoclonal antibody (tozorakimab) [15] or via soluble ST2 (sST2) enhanced wound closure in cultured primary normal human bronchial epithelial (NHBE) cells (figure 1a and supplementary figure S1a). In contrast, ST2-neutralising antibodies (supplementary figure S1b) had no effect on wound closure in NHBE cells or a lung epithelial cell line (A549 cells) (figure 1a and supplementary figure S1a, c). Gene expression analysis revealed that transcripts encoding sST2 were present in NHBE cells, but full-length ST2 (ST2L) was not detected (supplementary figure S1d, e). In corroboration with the prior literature, ST2 was not detected in A549 cells (supplementary figure S1d, e) [16]. Additionally, IL-33 was unable to induce NF-ĸB activation and IL-6 release in A549 cells unless they were transduced with ST2L (supplementary figure S1f–h). These findings suggest that IL-33 can regulate epithelial functions via an ST2-independent mechanism.
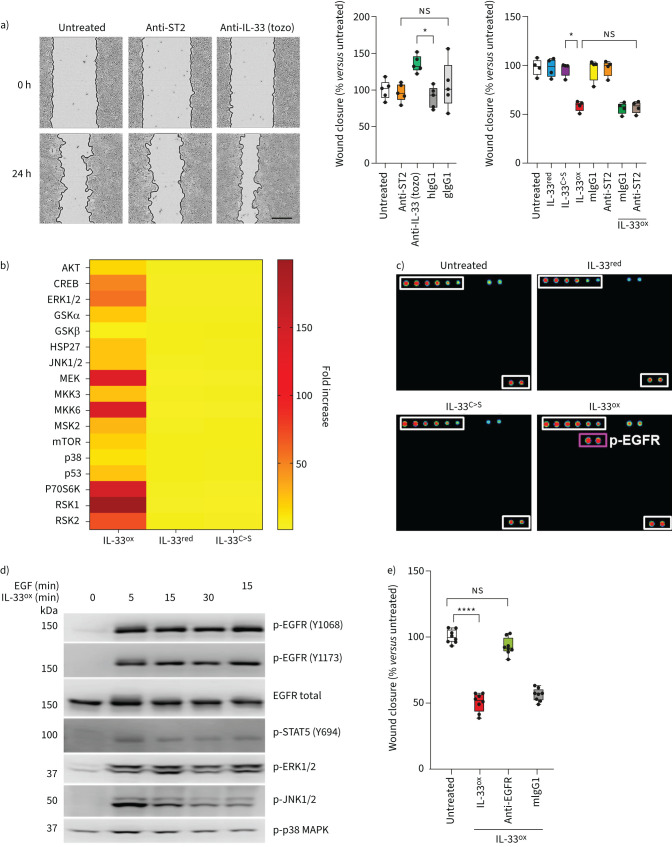
FIGURE 1.
Oxidised interleukin 33 (IL-33ox) promotes the activation of epidermal growth factor receptor (EGFR). a) Representative images of scratch wound closure in submerged cultures at 0 h and 24 h shown on left (scale bar: 300 μm) and plotted as percentage wound closure at 24 h (middle) in growth factor-starved submerged normal human bronchial epithelial (NHBE) cells following treatment with serum stimulation-2 (ST2)-neutralising antibody (goat), IL-33-neutralising antibody (tozorakimab (tozo)) or human (hIgG1) or goat (gIgG1) immunoglobulin G1 isotype control antibody versus untreated control (five individual donors). Right, scratch wound closure in submerged NHBE cells at 24 h following treatment with reduced IL-33 (IL-33red), oxidation-resistant mutant IL-33 (IL-33C>S), IL-33ox, mouse immunoglobulin G1 (mIgG1) isotype control antibody, ST2-neutralising antibody, IL-33ox+mIgG1 isotype control antibody or IL-33ox+ST2-neutralising antibody versus untreated control (four individual donors). b) Phospho-proteome profiler MAPK signalling array analysis in submerged NHBE cells treated with 30 ng·mL−1 of IL-33ox, IL-33red or IL-33C>S for 10 min (one donor). c) Phospho-tyrosine kinase receptor array analysis in growth factor-starved submerged NHBE cells treated with 30 ng·mL−1 of IL-33red, IL-33C>S or IL-33ox for 10 min versus untreated control (one donor). p-EGFR: phosphorylated epidermal growth factor receptor. d) Western blot analysis of p-EGFR, total EGFR, p-STAT5, p-ERK1/2, p-JNK1/2 and p-p38 MAPK over time in submerged NHBE cells following stimulation with 30 ng·mL−1 of epidermal growth factor (EGF) or IL-33ox. e) Scratch wound closure in growth factor-starved submerged NHBE cells at 24 h following treatment with IL-33ox, IL-33ox+EGFR-neutralising antibody or IL-33ox+mIgG1 isotype control antibody versus untreated control (four individual donors in experimental replicate). The data shown are a representative example from at least two independent experiments. Individual data points are shown in panels a and e, and further details of box plots can be found in the supplementary materials; data are normalised to untreated control. *: p≤0.05; ****: p≤0.0001 (non-parametric Kruskal–Wallis test with multiple comparisons). ns: not significant.
IL-33ox impairs wound closure via EGFR
The effect of the different forms of IL-33 (IL-33red and IL-33ox) on wound closure was assessed next. Wild-type IL-33red and an oxidation-resistant mutant form of IL-33 (IL-33C>S) had no significant effect on wound closure in NHBE cells (figure 1a), despite clear ST2-dependent activity as evidenced by the ability to induce NF-ĸB translocation in ST2-expressing human umbilical vein endothelial cells (supplementary figure S2a). In contrast, IL-33ox decreased wound closure in both NHBE cells (figure 1a) and A549 cells (supplementary figure S2b, c), which do not express ST2 or show ST2-dependent NF-ĸB translocation activity [16]. In addition, ST2 neutralisation was unable to reverse the decrease in wound closure produced by IL-33ox (figure 1a and supplementary figure S2b). Wild-type IL-33red, which retains the ability to become oxidised, decreased wound closure in NHBE cells at much higher concentrations than IL-33ox (supplementary figure S2d).
A signalling array demonstrated that IL-33ox activates multiple molecules downstream of receptor tyrosine kinase (RTK) pathways in NHBE cells (figure 1b). RTK phosphorylation arrays identified that epidermal growth factor receptor (EGFR) was activated by IL-33ox (figure 1c), and homogeneous time-resolved fluorescence confirmed that IL-33ox increased EGFR phosphorylation in a dose-dependent manner (supplementary figure S2e). Similar to epidermal growth factor (EGF), IL-33ox led to the phosphorylation of signalling molecules downstream of EGFR (STAT5, JNK and ERK1/2) (figure 1d and supplementary figure S2f, g). Neutralisation of EGFR prevented the effects of IL-33ox on scratch wound closure (figure 1e). IL-33ox also reduced cell proliferation and, consistent with previous studies [17], exogenous EGF elicited opposite effects, leading to increased wound closure and cell proliferation (supplementary figure S2h, i). These data suggest that although both EGF and IL-33ox activate EGFR, they have different functional outcomes. When both ligands (30 ng·mL−1) were added to the epithelial scratch wound assay simultaneously, the healing response returned to baseline (supplementary figure S2j). The balance of these ligands may be important for fine-tuning EGFR-mediated biological effects.
IL-33ox binds RAGE to signal via EGFR
We performed immunoprecipitation and mass spectrometry on NHBE cells to investigate whether additional molecules were involved in the IL-33ox–EGFR signalling complex (figure 2a). Receptor for advanced glycation end products (RAGE) was the only surface molecule that immunoprecipitated with EGFR in an IL-33ox-dependent manner (figure 2a, b and supplementary table S1). The existence of the IL-33ox–RAGE/EGFR complex was confirmed by immunoprecipitation of EGFR after stimulation with IL-33ox in A549 cells (figure 2bi). An IL-33ox–EGFR complex did not form in RAGE knockout cells (figure 2bii), suggesting that RAGE is a primary receptor for IL-33ox.
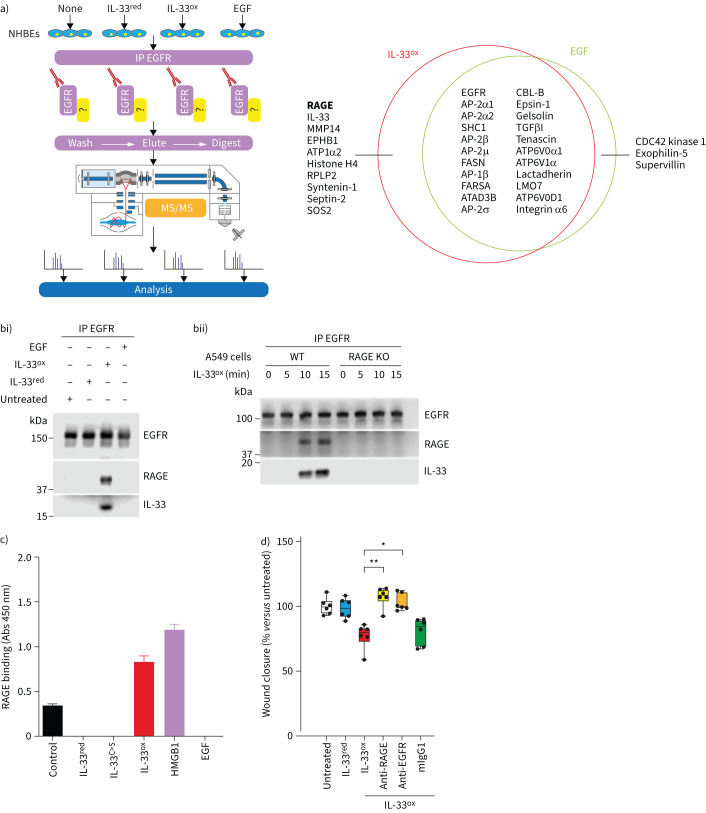
FIGURE 2.
Receptor for advanced glycation end products (RAGE) binds to oxidised interleukin 33 (IL-33ox), enabling the formation of a complex with epidermal growth factor receptor (EGFR). a) Left, schematic showing methodology for immunoprecipitation (IP) of EGFR in submerged normal human bronchial epithelial (NHBE) cells and tandem mass spectrometry (MS/MS). Right, quantitative Venn diagram showing the overlap of proteins detected by MS/MS following IP of EGFR in submerged NHBE cells treated with IL-33ox or epidermal growth factor (EGF) for 10 min. Proteins with a total unique peptide count of ≥3 in either treatment group are shown. Proteins overlapping with untreated control and IL-33ox are not shown. b) Western blot analysis of i) EGFR, RAGE and IL-33 following IP of EGFR in submerged NHBE cells treated with reduced IL-33 (IL-33red), IL-33ox or EGF for 10 min versus untreated control (one individual donor); ii) EGFR, RAGE and IL-33 following IP of EGFR in A549 cells or RAGE knockout (KO) A549 cells treated with IL-33ox for 0, 5, 10 or 15 min. c) Direct ELISA detecting binding of RAGE-Fc to immobilised IL-33red, oxidation-resistant mutant IL-33 (IL-33C>S), IL-33ox, high mobility group box 1 (HMGB1) or EGF. Absorption at 450 nm is shown. Error bars show sem. d) Scratch wound closure in submerged NHBE cells at 24 h following treatment with IL-33red, IL-33ox, IL-33ox+RAGE-neutralising antibody, IL-33ox+EGFR-neutralising antibody or IL-33ox+mouse immunoglobulin G1 (mIgG1) isotype control antibody versus untreated control (six individual donors). The data shown in b–d are representative examples from at least two independent experiments. Individual data points are shown, and further details of box plots can be found in the supplementary materials; data are normalised to untreated control. *: p≤0.05; **: p≤0.01 (non-parametric Kruskal–Wallis test with multiple comparisons).
These interactions were confirmed using biochemical binding assays. Both IL-33ox and the well-described RAGE ligand high mobility group box 1 (HMGB1), but not EGF, bound directly to RAGE (figure 2c), whereas IL-33red and IL-33C>S exclusively engaged with ST2 (supplementary figure S3a). In contrast to EGF, IL-33ox was able to form a complex with EGFR only when RAGE was present (supplementary figure S3b). In line with these findings, IL-33ox induced phosphorylation of EGFR and ERK1/2 to a similar extent as EGF, whereas other RAGE ligands did not. The low-affinity EGFR ligand amphiregulin displayed an EGFR response at much higher concentrations than IL-33ox or EGF (supplementary figure S3c, d).
Lastly, IL-33ox did not have an effect on wound closure in RAGE knockout cells (supplementary figure S3e), and anti-RAGE or anti-EGFR blocked the effects of IL-33ox on wound closure (figure 2d). These data suggest that IL-33ox binds to RAGE and forms a signalling complex with EGFR that modulates epithelial cell function (supplementary figure S3f).
IL-33ox causes mucin hypersecretion
To investigate how IL-33ox drives functional changes in lung epithelium, NHBE cells from healthy donors were differentiated into air–liquid interface (ALI) cultures (figure 3a), modelling human epithelial physiology [18]. IL-33ox treatment induced a plethora of transcriptional changes, in contrast to no treatment or treatment with IL-33C>S (figure 3b and supplementary figure S4a). ALI cultures were maintained in standard PneumaCult medium (containing 0.5 ng·mL−1 EGF), which potentially explains the limited number of differentially expressed genes observed with EGF treatment (figure 3b). IL-33ox decreased the expression of genes associated with epithelial differentiation and increased the expression of genes associated with negative regulation of wound closure (supplementary figure S4b). Genes associated with mitochondrial organisation, ATP metabolism, endoplasmic reticulum/Golgi vesicle transport and cellular stress markers were also upregulated, suggesting IL-33ox drives dramatic epithelial changes that require considerable metabolic resources. IL-33ox modulated multiple genes critical to mucociliary clearance, including those involved in extracellular matrix remodelling, carbohydrate biosynthesis, the secretory process and mucin production (supplementary figure S4b and supplementary table S2).
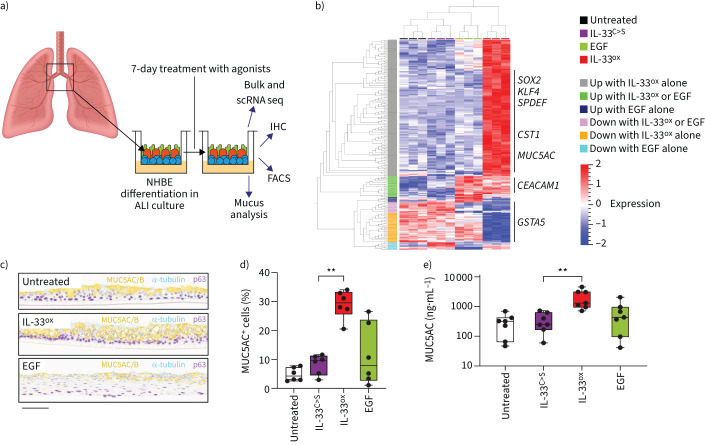
FIGURE 3.
Chronic oxidised interleukin 33 (IL-33ox) exposure induces an epithelial mucin hypersecretion phenotype. a) Schematic representation of bronchial epithelial air–liquid interface (ALI) cultures and end-point assays. FACS: fluorescence-activated cell sorting; IHC: immunohistochemistry; NHBE: normal human bronchial epithelial; scRNA seq: single-cell RNA sequencing. b) Heat map showing expression levels of genes in bronchial ALI cultures with significant variation between untreated control and oxidation-resistant mutant IL-33 (IL-33C>S), epidermal growth factor (EGF) and IL-33ox treatments from bulk RNA sequencing (three individual donors) (ANOVA, false discovery rate <0.0001). c) Representative IHC of healthy bronchial ALI cultures following a 7-day treatment with 30 ng·mL−1 of IL-33ox or EGF versus untreated control: mucin 5AC/B (MUC5AC/B) for goblet cells (yellow), acetylated α-tubulin for ciliated cells (teal) and p63 for basal cells (purple) (scale bar: 70 µm). d) Quantification of MUC5AC single-positive goblet cells as judged by flow cytometry in bronchial ALI cultures (six individual donors). e) ELISA of MUC5AC secreted into the apical region of healthy bronchial ALI cultures stimulated with IL-33C>S, IL-33ox or EGF versus untreated control (seven individual donors). **: p≤0.01 (non-parametric Kruskal–Wallis test with multiple comparisons).
In ALI cultures, immunohistochemistry co-staining of the major gel-forming mucins showed a significant increase in mucin staining (figure 3c and supplementary figure S4c) that was predominantly due to an upregulation of mucin 5AC (MUC5AC), as shown by mRNA and protein levels (figure 3d and supplementary figure S4d–f). Increased MUC5AC secretion was corroborated by ELISA (figure 3e). Interestingly, upregulation of the gut gel-forming mucin MUC2 was also observed with IL-33ox stimulation (supplementary figure S4g), similar to IL-13-challenged airway epithelium. Importantly, blocking the IL-33ox–RAGE/EGFR pathway with anti-RAGE and anti-EGFR downregulated mucin expression; however, anti-EGF had no effect (supplementary figure S4h), confirming that EGF is not required for the observed increase in mucin expression [19]. These data show that the IL-33ox–RAGE/EGFR pathway promotes extensive epithelial changes, redirecting the airway epithelium towards mucin hypersecretion.
IL-33ox redirects epithelial cell fate
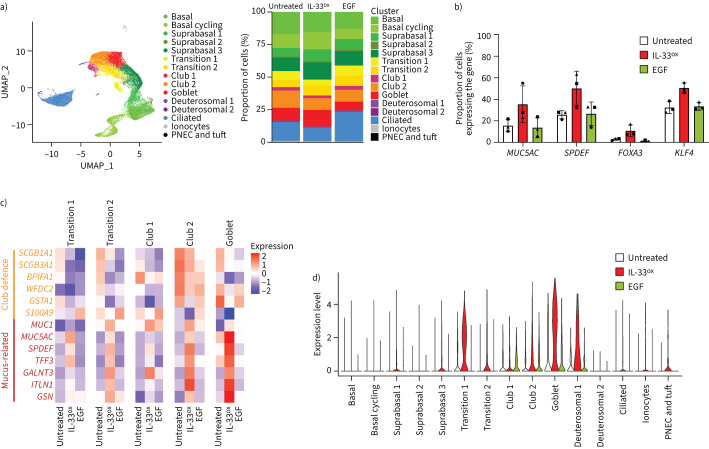
Next, we investigated the transcriptional changes induced by IL-33ox in ALI cultures derived from healthy donors at the single-cell level. We identified 15 cell states/types in healthy untreated ALI cultures that represented the major airway epithelial cell types and were consistent with the complex cell heterogeneity observed in vivo (figure 4a, supplementary table S3 and supplementary figure S5a–c) [20]. IL-33ox was associated with a slight increase in the proportion of goblet cells, at the expense of club and ciliated cells (figure 4a). In contrast, EGF increased the ciliated compartment at the expense of the other cell types (figure 4a). Most strikingly, IL-33ox increased the proportion of cells expressing MUC5AC and key transcription factors involved in goblet cell differentiation (SPDEF, FOXA3 and KLF4 [21, 22]) (figure 4b and supplementary figure S6a, b). IL-33ox also upregulated genes associated with MUC5AC expression and MUC5AC secretion [23] in goblet cells (supplementary figure S6b) and increased the expression of MUC5AC and related genes in secretory and non-secretory cells (figure 4c, d and supplementary figure S6c).
FIGURE 4.
Single-cell gene expression analysis identifies cell state changes supporting mucus hypersecretion. a) Left, UMAP plot of healthy bronchial epithelial air–liquid interface (ALI) cultures (three individual donors; 43 150 cells/single-cell transcriptomes) displaying the annotated cell states/types. Right, visual representation of changes in the proportions of cell states/types in bronchial ALI cultures following 7-day treatment with oxidised interleukin 33 (IL-33ox) or epidermal growth factor (EGF) (30 ng·mL−1) versus untreated control. b) Changes in the percentages of cells expressing mucin-related genes (MUC5AC, SPDEF, FOXA3 and KLF4) in bronchial ALI cultures after treatment with IL-33ox or EGF versus untreated control. Each symbol represents one healthy donor. Error bars show sem. c) Heat map showing the scale-normalised average expression levels of genes associated with mucin production or defence in the secretory states in bronchial ALI cultures treated with IL-33ox or EGF versus untreated control. d) Expression levels of MUC5AC in the annotated cell states/types in healthy bronchial ALI cultures treated with IL-33ox or EGF versus untreated control. PNEC: pulmonary neuroendocrine cell.
Differential gene expression analysis showed that club cell-associated genes involved in epithelial defence functions (e.g. SCGB1A1, SCGB3A1, BPIFA1, WFDC2, GSTA1 and S100A9 [24–26]) were downregulated in all secretory cells, whereas mucus-related genes (e.g. MUC5AC, MUC1, TFF3, GALNT3, ITLN1 and GSN [23, 27]) were highly expressed after IL-33ox treatment (figure 4c and supplementary figure S6c). Notably, the most highly differentially expressed genes in the single-cell analysis were consistent with data from bulk RNA sequencing (supplementary figure S4b and supplementary figure S6d, e). Collectively, these data suggest that IL-33ox drives epithelial remodelling, promoting a mucin hypersecretion phenotype at the expense of club- and cilia-related genes (supplementary figure S6f–i).
Blocking IL-33 reverses key COPD features
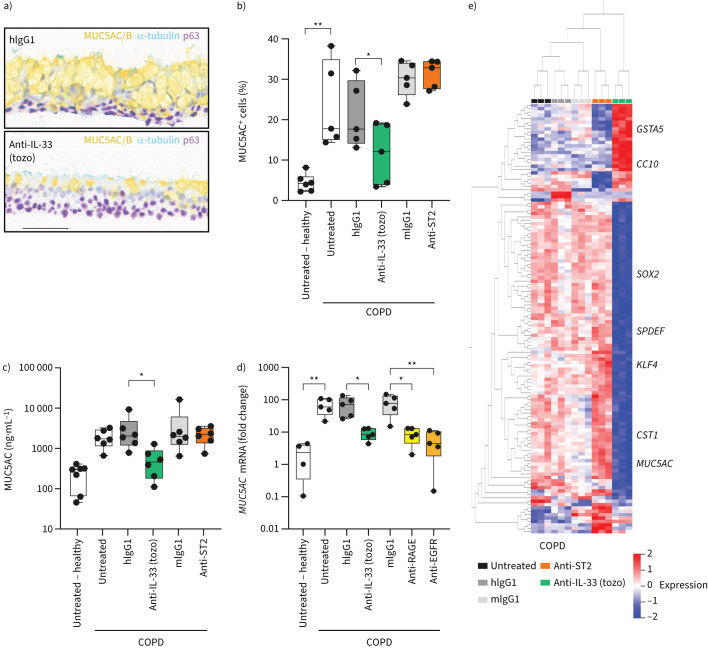
Defective wound healing and epithelial changes induced by chronic exposure to exogenous IL-33ox were reminiscent of characteristics observed in airway epithelial cells derived from patients with COPD [4, 28, 29]. Inhibition of IL-33 reversed defective wound healing in submerged COPD cultures (supplementary figure S7a) and reduced the proportion of mucus-secreting cells and the amount of MUC5AC released in COPD ALI cultures (figure 5a–c and supplementary figure S7b–e). No changes were observed after selectively blocking ST2 (figure 5b, c), indicating that an ST2-independent IL-33ox pathway may drive this phenotype. Further supporting this hypothesis, ST2L was not detectable in COPD ALI cultures (supplementary figure S7f).
FIGURE 5.
Inhibition of endogenous oxidised interleukin 33 (IL-33ox) reduces chronic obstructive pulmonary disease (COPD) epithelial dysfunction and mucus hypersecretion. a) Representative immunohistochemistry of COPD bronchial epithelial air–liquid interface (ALI) cultures following treatment with IL-33-neutralising antibody (tozorakimab (tozo)) or human immunoglobulin G1 (hIgG1) isotype control antibody (1 μg·mL−1) for 7 days: mucin 5AC/B (MUC5AC/B) for goblet cells (yellow), acetylated α-tubulin for ciliated cells (teal) and p63 for basal cells (purple) (scale bar: 70 µm). b) Flow cytometry analysis of intracellular MUC5AC in dissociated healthy or COPD bronchial ALI cultures following treatment with tozorakimab, serum stimulation-2 (ST2)-neutralising antibody or the relevant isotype control antibody versus untreated control (six individual healthy and five individual COPD donors). c) ELISA of MUC5AC secreted into the apical region of healthy or COPD ALI cultures incubated with tozorakimab, ST2-neutralising antibody or the relevant isotype control antibody versus untreated control (seven individual healthy and six individual COPD donors). d) MUC5AC expression determined by reverse transcription quantitative PCR in healthy or COPD bronchial ALI cultures incubated with tozorakimab, receptor for advanced glycation end products (RAGE)-neutralising antibody, endothelial growth factor receptor (EGFR)-neutralising antibody or the relevant isotype control antibody versus untreated control (four individual healthy and five individual COPD donors). Single data points from individual donors are shown; data are normalised to one donor in the untreated control group. e) Heat map showing expression levels of genes with significant variation between untreated control and tozorakimab, ST2-neutralising or relevant isotype control treatments from bulk RNA sequencing (three individual donors) (ANOVA, false discovery rate <0.0001). Individual data points are shown in panels b–d, and further details of box plots can be found in the supplementary materials. mIgG1: mouse immunoglobulin G1. *: p≤0.05; **: p≤0.01 (non-parametric Kruskal–Wallis test with multiple comparisons).
We next explored the role of the RAGE/EGFR complex in driving mucin hypersecretion in COPD ALI cultures. RAGE, EGFR and IL-33 were localised in COPD lung airways (supplementary figure S7g) and detected in COPD ALI cultures (supplementary figure S7f, h–j). Blocking EGFR or RAGE in COPD epithelium reduced MUC5AC expression to the levels observed in healthy donors, mimicking the effects of neutralising IL-33 (figure 5d). Similar results were obtained in COPD small airway ALI cultures (supplementary figure S8a).
Importantly, dexamethasone (a corticosteroid used to treat acute COPD exacerbations [30]), anti-IL-13 and anti-IL-4R (both constituents of a key pathway in mucus secretion [20]) did not alter MUC5AC mRNA levels (supplementary figure S8b). Blockade of IL-13 signalling did not affect the ability of IL-33ox to upregulate mucins and, conversely, neutralising IL-33 did not prevent IL-13-induced upregulation of the mucin gene MUC2 (supplementary figure S8c, d). This suggests that IL-33ox acts independently of IL-13 and IL-4R to increase mucin hypersecretion.
Inhibition of IL-33ox signalling induced substantial transcriptomic changes, dampening the mucus cell phenotype and restoring genes associated with cilia and club cells (figure 5e and supplementary figure S9a, b). Expression of the key regulator of goblet cell differentiation, SPDEF [31], was dramatically reduced by neutralising IL-33 in COPD ALI cultures, reaching levels similar to those in healthy donors (figure 5e and supplementary figure S9c). We also observed downregulation of other genes known to be associated with goblet cell differentiation and carbohydrate biosynthesis, and upregulation of genes associated with detoxification functions, club cells and cilia organisation and assembly (supplementary figure S9b). Importantly, downregulated genes and associated functions observed after IL-33 inhibition overlapped significantly with upregulated genes induced by IL-33ox in healthy lung epithelium (supplementary figure S9d, e). This reciprocal gene expression profile supports a central role for IL-33ox in driving a COPD phenotype in ALI cultures.
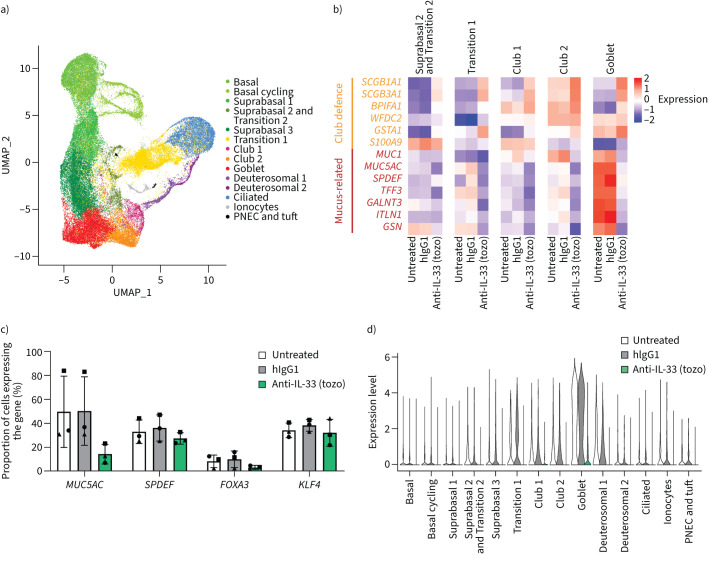
Using single-cell transcriptomics, we identified 15 cell states/types in COPD ALI cultures that correspond with those observed in healthy ALI cultures (supplementary figure S5a, b and supplementary figure S10a, b) and investigated the effects of blocking endogenous IL-33 signalling (figure 6a–d and supplementary figure S10a, b). Neutralisation of IL-33 resulted in a slight decrease in the proportion of goblet cells, accompanied by increases in club and transition 1 cell states/types (supplementary figure S11a). Differential gene expression analysis showed the upregulation of genes associated with epithelial host defence functions and club cell markers (e.g. SCGB1A1 and SCGB3A1 [26]) (figure 6b). Genes involved in mucus production (including MUC5AC translocation and secretion) and transcription factors that induce goblet cell differentiation were dramatically downregulated (figure 6b, supplementary figure S10a, b and supplementary figure S11b, c).
FIGURE 6.
Inhibition of oxidised interleukin 33 (IL-33ox) reverses chronic obstructive pulmonary disease (COPD)-related cell state changes at the single-cell level. a) UMAP plot of COPD bronchial epithelial air–liquid interface (ALI) cultures treated with IL-33-neutralising antibody (tozorakimab (tozo)) or isotype control antibody versus untreated control (three individual donors; 60 137 cells from all conditions), showing the annotated cell states/types. b) Heat map showing the scale-normalised average expression levels of genes associated with mucin production or defence in the secretory states in COPD bronchial ALI cultures treated with tozorakimab or human immunoglobulin G1 (hIgG1) isotype control antibody versus untreated control. c) Changes in the percentages of cells expressing mucin-related genes (MUC5AC, SPDEF, FOXA3 and KLF4) in COPD bronchial ALI cultures after treatment with tozorakimab or hIgG1 isotype control antibody versus untreated control. Each symbol represents one COPD donor. Error bars show sem. d) MUC5AC expression levels in the annotated cell states/types in COPD bronchial ALI cultures treated with tozorakimab or hIgG1 isotype control antibody versus untreated control.
IL-33 inhibition reduced the proportion of cells expressing MUC5AC to 14% of cells compared with untreated or isotype-treated controls (∼50% of cells) (figure 6c and supplementary figure S11b). Similarly, IL-33 inhibition reduced the expression of transcription factors governing goblet cell differentiation (e.g. SPDEF, FOXA3 and KLF4) (figure 6c and supplementary figure S11b). In COPD ALI cultures, non-secretory cell types expressed abnormally high levels of MUC5AC (figure 6d), similar to those observed in healthy ALI cultures treated with exogenous IL-33ox (figure 4d). Inhibition of IL-33 also suppressed MUC5AC expression in non-secretory cell types (figure 6d), reaching levels similar to those observed in healthy donors (figure 4d). Indeed, the gene expression changes observed following IL-33 neutralisation suggest a reduction in mucin hypersecretion and an increase in club cell function (supplementary figure S11b–f). These data indicate that inhibition of IL-33ox can reverse pathological features of COPD epithelium and restore aspects of a healthy epithelial phenotype (supplementary figure S11g, h).
IL-33ox gene signatures enriched in COPD
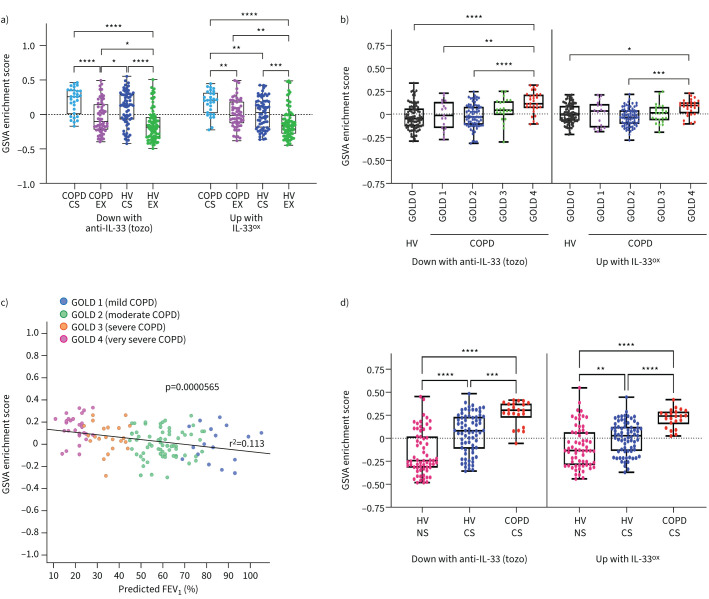
We used epithelial gene signatures identified in vitro from our bulk RNA sequencing analysis to understand the relevance of the IL-33ox–RAGE/EGFR pathway in patients with COPD. This comprised 135 (≥2-fold downregulated) genes from COPD ALI cultures following IL-33 inhibition, and 249 (≥2-fold upregulated) genes from healthy ALI cultures stimulated with IL-33ox (supplementary figure S9d and supplementary table S4). The most significant differentially expressed genes were selected; all had a false discovery rate adjusted p-value <0.05. Both signatures were applied to three independent, publicly available lung COPD cohorts (GSE37147, GSE47460 and GSE11784 [32–34]).
In the GSE37147 cohort, derived from bronchial brushes [32], both IL-33ox and anti-IL-33 gene signatures displayed an increased enrichment score associated with smoking and COPD status, with the highest signal found in current smokers with COPD (figure 7a). In the GSE47460 cohort, derived from lung homogenates [33], both signatures correlated with disease severity as assessed by gene enrichment according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages or the decline in predicted forced expiratory volume in 1 s (figure 7b, c). Lastly, in a cohort derived from brushes from small airways (GSE11784) [34], both signatures displayed a significantly increased enrichment score in COPD donors (figure 7d).
FIGURE 7.
Gene signatures defining how the effects of oxidised interleukin 33 (IL-33ox) in bronchial epithelial air–liquid interface (ALI) cultures correlate with disease severity in patients with chronic obstructive pulmonary disease (COPD). a) Gene set variation analysis (GSVA) of the tozorakimab (tozo) and IL-33ox signatures in the GSE37147 cohort samples. b) GSVA showing the correlation with disease severity in the GSE47460 cohort samples derived from lung explants, as assessed by gene enrichment according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages. c) GSVA showing the correlation of genes downregulated in COPD ALI cultures following IL-33-neutralising antibody (tozorakimab) treatment with disease severity (GOLD1–4) in the GSE47460 cohort samples, as assessed by gene enrichment score according to predicted forced expiratory volume in 1 s (FEV1). d) GSVA showing the correlation with smoking status and COPD in the GSE11784 cohort samples derived from lung explants. Data are plotted as mean±sem. Details of box plots can be found in the supplementary materials. CS: current smoker; EX: ex-smoker; HV: healthy volunteer; NS: non-smoker. *: p≤0.05; **: p≤0.01; ***: p≤0.001; ****: p≤0.0001 (one-way ANOVA with Tukey's honest significant difference test).
Discussion
We have identified a novel ST2-independent IL-33 epithelial signalling pathway that may impact COPD pathogenesis. Here, we show for the first time that IL-33ox signals via a complex of RAGE and EGFR in the airway epithelium. Activation of the IL-33ox–RAGE/EGFR pathway results in a mucus-producing phenotype; moreover, mucus-related gene expression pathways were activated in club cells, at the expense of epithelial defence genes. Expression of mucus-related pathways was also elevated in ciliated cells, which could result in cell malfunction [20]. This increase in mucus-producing cells may result in inflammation and obstruction of the airways [35], contributing to the irreversible epithelial changes observed in COPD.
Previous studies indicate that inhaled stimuli cause rapid changes to the airway epithelium through an IL-33-mediated mechanism [8, 36, 37]. Dysregulation of IL-33ox signalling might cause excessive epithelial remodelling, mucin hypersecretion and muco-ciliary arrest, increasing the exacerbation risk. Many of the effects of IL-33ox on epithelial function overlap with known effects of IL-13 [20], but occur in an IL-13-independent manner. This overlapping biology raises the possibility that IL-33ox could be an alternative driver of epithelial pathogenesis in chronic inflammatory diseases.
IL-33, RAGE and EGFR have each been individually linked to COPD and the severity of COPD [7, 8, 38]. We have identified that these molecules act in a single pathway and may fine-tune EGFR function to alter the airway epithelial architecture, adding to the complexity of EGFR signalling [39]. Previous data indicate that different EGFR ligands may modulate the balance of receptor degradation or recycling [40] and stimulate distinct patterns of EGFR tyrosine phosphorylation [41], which may promote specific downstream signalling. Further work will be required to understand how IL-33ox modulates EGFR signalling and how the role of the IL-33ox–RAGE/EGFR pathway intersects with the function of each molecule in their other known signalling pathways. Indeed, RAGE is a promiscuous receptor that binds multiple danger-signalling ligands; furthermore, both RAGE and many of its ligands have been shown to be upregulated in patients with COPD [42]. Activation of RAGE results in increased inflammation, mucus production and oxidative stress [42].
Oxidative stress is also known to be increased in COPD: activated macrophage and neutrophil numbers are higher in the lungs of patients with COPD than in healthy controls. These cells release large amounts of reactive oxygen species, particularly during exacerbations [43]. Oxidative stress has been shown to upregulate IL-33 expression in the airway epithelium following viral infection [44]. Furthermore, this oxidative environment might alter the balance of IL-33red and IL-33ox. Another factor that may regulate IL-33ox signalling is the availability of sST2, an endogenous antagonist of IL-33 [45], which may prevent oxidation of IL-33 as well as IL-33red binding to ST2 [15]. We found that levels of sST2 were lower in COPD ALI cultures than in healthy ALI cultures. sST2 has also been shown to be reduced in bronchoalveolar lavage fluid from patients with COPD [46]. Overall, oxidative stress combined with an imbalance of sST2 may promote a cycle of IL-33 release, uncontrolled inflammation and aberrant epithelial remodelling.
The analysis of the molecules involved in the modulation of the IL-33red and IL-33ox pathways in patients with COPD will help us to better select a population responsive to anti-IL-33 therapies. Given that these data indicate that IL-33ox inhibition can reverse COPD-associated epithelial pathological features, we speculate that therapies designed to inhibit signalling of both IL-33ox and IL-33red will have a greater clinical impact than those targeting IL-33red/ST2-induced inflammation alone. Tozorakimab directly inhibits IL-33red/ST2 signalling and indirectly inhibits IL-33ox–RAGE/EGFR signalling by preventing the formation of IL-33ox [15]. The efficacy and safety of tozorakimab is being explored in phase 2 and 3 trials for several inflammatory diseases, including COPD (NCT04631016, NCT05166889, NCT05158387), acute respiratory failure (NCT05624450), COVID-19 (EudraCT: 2020-001736-95), asthma (NCT04570657) and diabetic kidney disease (NCT04170543).
There are several limitations to this study. First, although two- and three-dimensional epithelial cultures model the in vivo system, the lung mucosa is highly complex and COPD lungs have dysfunction in a broad range of pathways and cell types. Therefore, modulating the activity of an individual pathway may not be sufficient to restore lung function. Second, it is difficult to evaluate which factors determine the balance between IL-33red and IL-33ox and therefore to establish the contribution of IL-33ox to the pathology of COPD or other inflammatory conditions. Third, further investigations are needed to understand how EGF family members and IL-33ox balance epithelial functions through EGFRs.
Overall, our data expand the role of RAGE as a broad sensor of cell injury to include a novel form of an alarmin, IL-33ox, and elucidate a new role of EGFR in signal transduction as part of a previously unappreciated tripartite receptor complex (IL-33ox–RAGE/EGFR). This study contributes to our understanding of RAGE and EGFR biology and prompts us to review past data under a new lens. Further studies are warranted to clarify the role of the IL-33ox–RAGE/EGFR pathway in health, chronic disease and infection.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02210-2022.Supplement (3.5MB, pdf)
Shareable PDF
Acknowledgements
We thank Elizabeth Haughey, Hannah Tompkins, Aleksandra Betiuk, Jayesh Majithiya (Respiratory, Inflammation & Autoimmunity, AstraZeneca, Cambridge, UK), Amy Napier, Elizabeth Owen, Claire Hollins (Bioscience Asthma & Skin Immunity, Research and Early Development Respiratory & Immunology, BioPharmaceuticals R&D, AstraZeneca, Cambridge, UK), Stephanie Heasman (Imaging & AI, Clinical Pharmacology & Safety Sciences, BioPharmaceuticals R&D, AstraZeneca, Cambridge, UK), Dominic Corkill (Bioscience In Vivo, Research and Early Development, Respiratory & Immunology, BioPharmaceuitcals R&D, AstraZeneca, Cambridge, UK), Trevor Wilkinson (Biologics Engineering, Oncology R&D, AstraZeneca, Cambridge, UK) and Rebecca Weissbach (Antibody Discovery and Protein Engineering, AstraZeneca, Cambridge, UK) for the support provided. We thank Paul Davies and Renata Soares (MRC-PPU, University of Dundee, Dundee, UK) for proteomic analysis. We also acknowledge Natasha Rangwani and Kelly Soady (PharmaGenesis London, London, UK) for medical writing support, which was funded by AstraZeneca.
Footnotes
Author contributions: S. Strickson conceived the study, designed the study and experiments, conducted experiments, and acquired and analysed data. K.F. Houslay conceived the study, designed the study and experiments, conducted experiments, and acquired and analysed data. V.A. Negri analysed data. Y. Ohne analysed data. T. Ottosson analysed data. R.B. Dodd conducted experiments. C.C. Huntington conducted experiments. T. Baker analysed data. J. Li conducted experiments and acquired and analysed data. K.E. Stephenson conducted experiments and acquired and analysed data. A.J. O'Connor conducted experiments and acquired and analysed data. J.S. Sagawe conducted experiments and acquired and analysed data. H. Killick conducted experiments and acquired and analysed data. T. Moore conducted experiments. D.G. Rees conducted experiments. S. Koch conducted experiments and acquired data. C. Sanden conducted experiments. Y. Wang analysed data. E. Gubbins conducted experiments. M. Ghaedi designed the study and experiments and analysed data. R. Kolbeck conceived the study and designed the study and experiments. S. Saumyaa conducted experiments and acquired and analysed data. J.S. Erjefält analysed data. G.P. Sims conducted experiments. A.A. Humbles conceived the study and designed the study and experiments. I.C. Scott conceived the study, designed the study and experiments, and acquired and analysed data. X. Romero Ros conceived the study and designed the study and experiments. E.S. Cohen conceived the study, designed the study and experiments, and analysed data. All authors were involved in drafting the manuscript, provided critical revisions for important intellectual content, approved the final version submitted for publication and agreed to be accountable for all aspects of the work.
This article has an editorial commentary: https://doi.org/10.1183/13993003.01301-2023
Conflict of interest: S. Strickson, V.A. Negri, Y. Ohne, T. Ottosson, R.B. Dodd, C.C. Huntington, T. Baker, J. Li, K.E. Stephenson, A.J. O'Connor, J.S. Sagawe, H. Killick, D.G. Rees, S. Koch, Y. Wang, M. Ghaedi, S. Saumyaa, G.P. Sims, I.C. Scott, X. Romero Ros and E.S. Cohen are employees of AstraZeneca and may hold stock or stock options in AstraZeneca. K.F. Houslay, T. Moore, E. Gubbins, R. Kolbeck and A.A. Humbles are former employees of AstraZeneca and may hold stock or stock options in AstraZeneca. C. Sanden has nothing to disclose. J.S. Erjefält is a founder and board member of Medetect AB.
Support statement: AstraZeneca funded this study and participated in the study design, data collection, data analysis and data interpretation. AstraZeneca reviewed the publication, without influencing the opinions of the authors, to ensure medical and scientific accuracy and the protection of intellectual property. The corresponding author had access to all data in the study and had the final responsibility to submit the manuscript for publication. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Carlier FM, de Fays C, Pilette C. Epithelial barrier dysfunction in chronic respiratory diseases. Front Physiol 2021; 12: 691227. doi: 10.3389/fphys.2021.691227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol 2009; 4: 435–459. doi: 10.1146/annurev.pathol.4.110807.092145 [DOI] [PubMed] [Google Scholar]
- 3.Barnes PJ. COPD 2020: new directions needed. Am J Physiol Lung Cell Mol Physiol 2020; 319: L884–L886. doi: 10.1152/ajplung.00473.2020 [DOI] [PubMed] [Google Scholar]
- 4.Gohy S, Carlier FM, Fregimilicka C, et al. . Altered generation of ciliated cells in chronic obstructive pulmonary disease. Sci Rep 2019; 9: 17963. doi: 10.1038/s41598-019-54292-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabe KF, Celli BR, Wechsler ME, et al. . Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med 2021; 9: 1288–1298. doi: 10.1016/S2213-2600(21)00167-3 [DOI] [PubMed] [Google Scholar]
- 6.Smith D, Helgason H, Sulem P, et al. . A rare IL-33 loss-of-function mutation reduces blood eosinophil counts and protects from asthma. PLoS Genet 2017; 13: e1006659. doi: 10.1371/journal.pgen.1006659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byers DE, Alexander-Brett J, Patel AC, et al. . Long-term IL-33–producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest 2013; 123: 3967–3982. doi: 10.1172/JCI65570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kearley J, Silver JS, Sanden C, et al. . Cigarette smoke silences innate lymphoid cell function and facilitates an exacerbated type I interleukin-33-dependent response to infection. Immunity 2015; 42: 566–579. doi: 10.1016/j.immuni.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 9.Gorska K, Nejman-Gryz P, Paplinska-Goryca M, et al. . Comparative study of IL-33 and IL-6 levels in different respiratory samples in mild-to-moderate asthma and COPD. COPD 2018; 15: 36–45. doi: 10.1080/15412555.2017.1416074 [DOI] [PubMed] [Google Scholar]
- 10.Miller AM. Role of IL-33 in inflammation and disease. J Inflamm (Lond) 2011; 8: 22. doi: 10.1186/1476-9255-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griesenauer B, Paczesny S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol 2017; 8: 475. doi: 10.3389/fimmu.2017.00475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaur D, Chachi L, Gomez E, et al. . ST2 expression and release by the bronchial epithelium is downregulated in asthma. Allergy 2020; 75: 3184–3194. doi: 10.1111/all.14436 [DOI] [PubMed] [Google Scholar]
- 13.Yagami A, Orihara K, Morita H, et al. . IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol 2010; 185: 5743–5750. doi: 10.4049/jimmunol.0903818 [DOI] [PubMed] [Google Scholar]
- 14.Cohen ES, Scott IC, Majithiya JB, et al. . Oxidation of the alarmin IL-33 regulates ST2-dependent inflammation. Nat Comm 2015; 6: 8327. doi: 10.1038/ncomms9327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.England E, Rees DG, Scott IC, et al. . Tozorakimab (MEDI3506): a dual-pharmacology anti-IL-33 antibody that inhibits IL-33 signalling via ST2 and RAGE/EGFR to reduce inflammation and epithelial dysfunction. bioRxiv 2023; preprint [ 10.1101/2023.02.28.527262]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jo S, Kim E, Kwak A, et al. . Reconstitution of ST2 (IL-1R4) specific for IL-33 activity; no suppression by IL-1Ra though a common chain IL-1R3 (IL-1RAcP) shared with IL-1. Cytokine 2016; 83: 33–40. doi: 10.1016/j.cyto.2016.03.013 [DOI] [PubMed] [Google Scholar]
- 17.Puddicombe SM, Polosa R, Richter A, et al. . Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J 2000; 14: 1362–1374. doi: 10.1096/fasebj.14.10.1362 [DOI] [PubMed] [Google Scholar]
- 18.Barron SL, Saez J, Owens RM. In vitro models for studying respiratory host–pathogen interactions. Adv Biol (Weinh) 2021; 5: e2000624. doi: 10.1002/adbi.202000624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tyner JW, Kim EY, Ide K, et al. . Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest 2006; 116: 309–321. doi: 10.1172/JCI25167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson ND, Everman JL, Chioccioli M, et al. . Single-cell and population transcriptomics reveal pan-epithelial remodeling in type 2-high asthma. Cell Rep 2020; 32: 107872. doi: 10.1016/j.celrep.2020.107872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park KS, Korfhagen TR, Bruno MD, et al. . SPDEF regulates goblet cell hyperplasia in the airway epithelium. J Clin Invest 2007; 117: 978–988. doi: 10.1172/JCI29176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen G, Korfhagen TR, Karp CL, et al. . Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity. Am J Respir Crit Care Med 2014; 189: 301–313. doi: 10.1164/rccm.201306-1181OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang G, Xu Z, Wang R, et al. . Genes associated with MUC5AC expression in small airway epithelium of human smokers and non-smokers. BMC Med Genomics 2012; 5: 21. doi: 10.1186/1755-8794-5-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akram KM, Moyo NA, Leeming GH, et al. . An innate defense peptide BPIFA1/SPLUNC1 restricts influenza A virus infection. Mucosal Immunol 2018; 11: 71–81. doi: 10.1038/mi.2017.45 [DOI] [PubMed] [Google Scholar]
- 25.Goldfarbmuren KC, Jackson ND, Sajuthi SP, et al. . Dissecting the cellular specificity of smoking effects and reconstructing lineages in the human airway epithelium. Nat Commun 2020; 11: 2485. doi: 10.1038/s41467-020-16239-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laucho-Contreras ME, Polverino F, Gupta K, et al. . Protective role for club cell secretory protein-16 (CC16) in the development of COPD. Eur Respir J 2015; 45: 1544–1556. doi: 10.1183/09031936.00134214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann W. Trefoil factor family (TFF) peptides and their diverse molecular functions in mucus barrier protection and more: changing the paradigm. Int J Mol Sci 2020; 21: 4535. doi: 10.3390/ijms21124535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boucher RC. Muco-obstructive lung diseases. N Engl J Med 2019; 380: 1941–1953. doi: 10.1056/NEJMra1813799 [DOI] [PubMed] [Google Scholar]
- 29.Perotin JM, Adam D, Vella-Boucaud J, et al. . Delay of airway epithelial wound repair in COPD is associated with airflow obstruction severity. Respir Res 2014; 15: 151. doi: 10.1186/s12931-014-0151-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. 2021. Available from https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf
- 31.Whitsett JA. Airway epithelial differentiation and mucociliary clearance. Ann Am Thorac Soc 2018; 15: S143–S148. doi: 10.1513/AnnalsATS.201802-128AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steiling K, van den Berge M, Hijazi K, et al. . A dynamic bronchial airway gene expression signature of chronic obstructive pulmonary disease and lung function impairment. Am J Respir Crit Care Med 2013; 187: 933–942. doi: 10.1164/rccm.201208-1449OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan J, Tedrow JR, Dutta JA, et al. . Expression of RXFP1 is decreased in idiopathic pulmonary fibrosis. Implications for relaxin-based therapies. Am J Respir Crit Care Med 2016; 194: 1392–1402. doi: 10.1164/rccm.201509-1865OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tilley AE, O'Connor TP, Hackett NR, et al. . Biologic phenotyping of the human small airway epithelial response to cigarette smoking. PLoS One 2011; 6: e22798. doi: 10.1371/journal.pone.0022798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hogg JC, Chu F, Utokaparch S, et al. . The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 2645–2653. doi: 10.1056/NEJMoa032158 [DOI] [PubMed] [Google Scholar]
- 36.Jackson DJ, Makrinioti H, Rana BM, et al. . IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med 2014; 190: 1373–1382. doi: 10.1164/rccm.201406-1039OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Y-H, Lai AC-Y, Chi P-Y, et al. . Pulmonary IL-33 orchestrates innate immune cells to mediate respiratory syncytial virus-evoked airway hyperreactivity and eosinophilia. Allergy 2020; 75: 818–830. doi: 10.1111/all.14091 [DOI] [PubMed] [Google Scholar]
- 38.Saied EM, Bediwy AS. Expression of epidermal growth factor receptor (EGFR) in the bronchial epithelium of patients with chronic obstructive pulmonary disease (COPD). Eur Respir J 2011; 38: 3823. [Google Scholar]
- 39.Kennedy SP, Hastings JF, Han JZ, et al. . The under-appreciated promiscuity of the epidermal growth factor receptor family. Front Cell Dev Biol 2016; 4: 88. doi: 10.3389/fcell.2016.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lill NL, Douillard P, Awwad RA, et al. . The evolutionarily conserved N-terminal region of Cbl is sufficient to enhance down-regulation of the epidermal growth factor receptor. J Biol Chem 2000; 275: 367–377. doi: 10.1074/jbc.275.1.367 [DOI] [PubMed] [Google Scholar]
- 41.Gilmore JL, Scott JA, Bouizar Z, et al. . Amphiregulin-EGFR signaling regulates PTHrP gene expression in breast cancer cells. Breast Cancer Res Treat 2008; 110: 493–505. doi: 10.1007/s10549-007-9748-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma A, Kaur S, Sarkar M, et al. . The AGE–RAGE axis and RAGE genetics in chronic obstructive pulmonary disease. Clin Rev Allergy Immunol 2021; 60: 244–258. doi: 10.1007/s12016-020-08815-4 [DOI] [PubMed] [Google Scholar]
- 43.Barnes PJ. Oxidative stress-based therapeutics in COPD. Redox Biol 2020; 33: 101544. doi: 10.1016/j.redox.2020.101544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aizawa H, Koarai A, Shishikura Y, et al. . Oxidative stress enhances the expression of IL-33 in human airway epithelial cells. Respir Res 2018; 19: 52. doi: 10.1186/s12931-018-0752-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molofsky AB, Savage AK, Locksley RM. Interleukin-33 in tissue homeostasis, injury and inflammation. Immunity 2015; 42: 1005–1019. doi: 10.1016/j.immuni.2015.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Stefano A, Caramori G, Barczyk A, et al. . Innate immunity but not NLRP3 inflammasome activation correlates with severity of stable COPD. Thorax 2014; 69: 516–524. doi: 10.1136/thoraxjnl-2012-203062 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-02210-2022.Supplement (3.5MB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-02210-2022.Shareable (970KB, pdf)