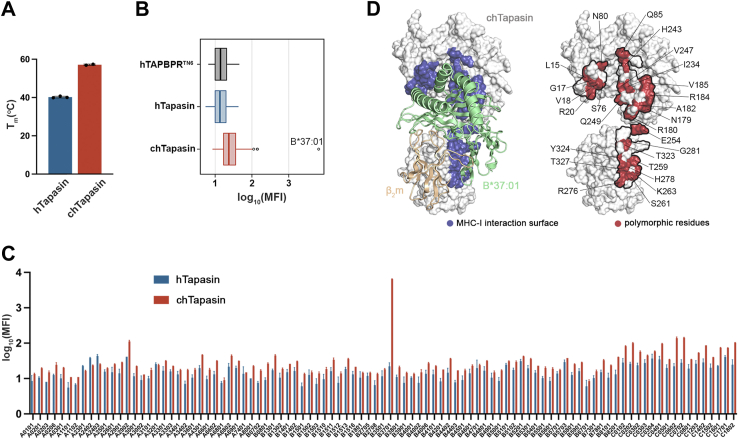
Figure 1.
The chicken Tapasin ortholog shows enhanced stability and distinct interactions with HLA allotypes.A, comparison of the thermal stabilities of human versus chicken Tapasin by differential scanning fluorimetry (DSF) experiments. Tm, melting temperature in degrees Celsius. The plotted data represent triplicate assays (n = 3). B, a Box plot of the distribution of human and chicken Tapasin MFI levels for 97 different MHC-I allotypes. Human TAPBPR carrying the substitutions E205L, R207E, Q209S, and Q272S (hTAPBPRTN6) was used as a negative control. The left boundary of the box represents the 25th percentile, the line within the box represents the median, and the right boundary of the box represents the 75th percentile. Whiskers extending above and below the box indicate the 10th and 90th percentiles, respectively. Any points appearing above the whiskers represent outliers that fall beyond the 90th percentile. Plotted data are mean from three replicates (n= 3). C, bar graph showing the binding levels of tetramerized hTapasin and chTapasin to 97 different HLA allotypes on the SABs plotted in (B). Data are mean ± SD of n = 3 independent experiments. D, surface representation of the chTapasin bound to HLA-B∗37:01 structure model generated using the BAKER-ROBETTA server (29). The predicted contact surfaces of chTapasin with the heavy chain of B∗37:01 are highlighted in blue (left). Surface representation of chTapasin where the polymorphic residues within the contact surfaces (denoted by the black line) are marked in red (right).