Abstract
To provide an economical magnetic photocatalyst and introduce an innovative approach for efficiently utilizing discarded waste rice noodle (WRN) and iron oxide scale (IOS), we initially converted WRN into carbon quantum dots (CQDs) using a hydrothermal method, simultaneously calcining IOS to obtain iron oxide (FeOx). Subsequently, we successfully synthesized a cost-effective, magnetic CQDs/FeOx photocatalytic composite for the first time by combining the resulting CQDs and FeOx. Our findings demonstrated that calcining IOS in an air atmosphere enhanced the content of photocatalytically active α-Fe2O3, while incorporating WRN-based CQDs into FeOx improved the electron-hole pair separation, resulting in increased O2 reduction and H2O oxidation. Under optimized conditions (IOS calcination temperature: 300 °C; carbon loading: 11 wt%), the CQDs/FeOx composite, utilizing WRN and IOS as its foundation, exhibited exceptional and reusable capabilities in photodegrading methylene blue and tetracycline. Remarkably, for methylene blue, it achieved an impressive degradation rate of 99.30% within 480 min, accompanied by a high degradation rate constant of 5.26 × 10−3 min−1. This composite demonstrated reusability potential for up to ten photocatalytic cycles without a significant reduction in the degradation efficiency, surpassing the performance of IOS and FeOx without CQDs. Notably, the composite exhibited strong magnetism with a saturation magnetization strength of 34.7 emu/g, which enables efficient and convenient recovery in photocatalytic applications. This characteristic is highly advantageous for the large-scale industrial utilization of photocatalytic water purification.
Keywords: waste rice noodle, iron oxide scale, carbon quantum dots/iron oxide composite, photocatalytic capability, magnetic
1. Introduction
In recent years, there has been growing interest in using semiconductor materials [1,2,3] as photocatalysts [4,5] for degrading low concentrations of organic and inorganic molecules in freshwater treatment [6], environmental remediation [7,8], industrial [9], and health applications [10,11]. Various types of semiconductors, such as TiO2 [12], ZnO [13], ZnS [14], CdS [15], and others, have been reported for photocatalytic decontamination over the past two decades. However, one major challenge encountered with most photocatalysts in powder form is their non-magnetic nature, making them difficult to separate from the purified solution. To address this issue, coupling [16,17,18,19] magnetic particles with non-magnetic semiconductor photocatalysts appears to be the most logical solution. By incorporating magnetic particles into the hybrid photocatalysts, an external magnetic field can be used to easily separate them after the photocatalytic process, ensuring reusability and offering a promising approach for environmental pollution control. Among the various magnetic particles, iron oxides—such as Fe3O4 [16,20], α-Fe2O3 [21], β-Fe2O3 [22], γ-Fe2O3 [23], FeO [24], and spinel ferrites (MFe2O4 [25])—have garnered significant attention in the field of photocatalysis due to their low cost, strong magnetism, environmental friendliness, and stability. However, in comparison to classical photocatalysts like TiO2 and ZnO, iron oxides do not demonstrate outstanding photocatalytic efficiency. Consequently, it has become essential to explore certain degrees of doping or composite modification to enhance their performance [26,27,28]. Among these, the composition of carbon quantum dots (CQDs) has proven to be an effective means of enhancing the photocatalytic performance of iron oxide materials [29,30,31,32,33,34]. There have been reports demonstrating that carbon quantum dots/iron oxide photocatalytic materials can efficiently degrade water-soluble organic pollutants such as methylene blue. For instance, Sun et al. prepared quantum dots using glucose as a carbon source and combined them with commercially-available magnetic Fe3O4 nanoparticles to create CQDs/Fe3O4 composite material. This composite achieved an 83% degradation rate of alkaline methylene blue solution within 30 min [29]. Additionally, Zhang et al. reported the synthesis of CQDs/MIL-101(Fe)/g-C3N4 composite photocatalytic material, using FeCl3 as the iron source and citric acid and ethylenediamine as carbon sources. This composite achieved a photocatalytic rate of 99.3% for methylene blue within 120 min [34]. However, it is worth noting that the CQDs/iron oxide photocatalytic materials reported to date have typically used commercially available iron and carbon sources, resulting in relatively high synthesis costs, which may pose challenges for large-scale commercialization.
The improper disposal of cooking waste has the potential to introduce pollutants into the soil and water ecosystems, posing a considerable urban governance dilemma [35]. The contemporary methods employed for the management of cooking waste in commercial settings [36,37] predominantly encompass techniques like anaerobic digestion [38], aerobic composting [39], landfill deposition [40], incineration [41], and the creation of forage [42]. Despite enabling the large-scale industrial treatment of cooking waste, these strategies are accompanied by notable limitations [43], including substantial land utilization, substantial capital investment in equipment, diminished product profit margins, and the potential for generating secondary pollutants like greenhouse gas emissions and leachates. In order to address these issues, our previous investigation proposed a novel approach to utilizing cooking waste, specifically waste rice noodle (WRN), with starch as the main component. This approach involved first hydrothermal carbonizing the WRN to create a solution of CQDs, which was then combined with specific inorganic nano powders like TiO2 or ZnO to produce CQDs/TiO2 [44] or CQDs/ZnO [45] photocatalytic composites for water pollution control. The CQDs/inorganic oxide composites derived from WRN displayed remarkable photocatalytic degradation efficiency, outperforming commercial TiO2 or ZnO, particularly under visible light illumination, for a range of water-soluble dyes. This conversion strategy of WRN to CQDs/TiO2 [44] or CQDs/ZnO [45] composites added significant value and offered promising prospects for industrialization, potentially providing a new method for cooking waste recycling. However, although both catalysts could be recycled and reused multiple times without significantly reducing their photocatalytic degradation capacity, each recycling step requires careful centrifugation, washing, and drying. This process posed significant challenges when applied to large-scale wastewater treatment in practical settings.
On the other hand, iron oxide scale (IOS [46]) is formed on the surface of steel during the steel rolling process when the steel is rapidly cooled with water, resulting in iron-containing oxides. If not properly treated, significant amounts of IOS may be released into the environment, potentially causing severe pollution to water bodies, soil, and other natural surroundings [47]. At present, the primary method for handling waste IOS is to reintroduce it into the metallurgical industry, where it is used in the smelting of steel [48]. This well-established approach is suitable for large-scale production, but it requires significant investments in terms of funds, equipment, land, and labor. Furthermore, there is the possibility that waste IOS may contain Ca, Mg, P, and Si, providing an alternative pathway for reusing it as fertilizer [49]. However, such fertilizers can only utilize a small fraction of the effective components present in the IOS, leading to generally low fertilizer efficiency. Considering these limitations, it is essential to explore novel approaches that utilize waste IOS as a raw material to produce high-tech, high-value functional materials.
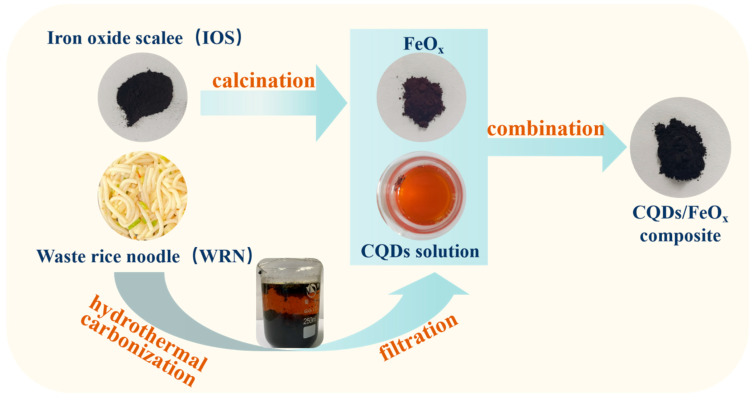
Based on previous research, our goal is to design and synthesize a new functional material derived from waste that boasts lower production costs and enhanced recyclability. We proposed integrating two types of waste materials, namely rice noodle waste (WRN) and iron oxide scale (IOS), as the raw materials. Firstly, the WRN underwent a hydrothermal process to convert it into CQDs, while the IOS was calcined to obtain iron oxides (FeOx). Subsequently, we combined the CQDs from WRN with the FeOx from IOS, achieving the pioneering synthesis of a cost-effective magnetic CQDs/FeOx photocatalytic composite material without the need for additional synthetic agents (see Figure 1). This material exhibited a good photocatalytic performance and could be effectively separated using magnets, showcasing its commendable recyclability and reusability. Our research delves into the material’s structure, photocatalytic performance, and photocatalytic mechanism, as well as thoroughly analyzing the effects of IOS calcination and CQDs combination on the material’s properties.
Figure 1.
Formation process of CQDs/FeOx composite using WRN and IOS as raw materials.
2. Experiment Section
2.1. Materials
The waste rice noodle (WRN) was obtained from the canteen located at the Guilin University of Technology in Guilin, China, with its primary organic components including starch (21.36 g/100 g), protein (1.91 g/100 g), and fat (0.4 g/100 g). The waste iron oxide scale (IOS, 200 mesh, main element content: Fe 71.09 wt%, O 27.41 wt%, Si 0.47 wt%, Ca 0.42 wt%, Mn 0.38 wt%, Al 0.23 wt%) was obtained from Valin Lianyuan Iron and Steel Co. Ltd. in Hunan, China. The tetracycline hydrochloride (98% purity), methylene blue (98.5% purity), nano ZnO (99% purity), nano TiO2 (99% purity), 1,4-benzoquinone (BQ, 98% purity), 2-propanol (IPA, 99% purity), ethylenediaminetetraacetic acid disodium salt (EDTA–2Na, 98% purity), as well as dimethyl pyridine N-oxide (DMPO, 99% purity), were procured from Macklin Reagent (Shanghai, China) and were used as received, without undergoing additional purification steps.
2.2. Synthesis
To initiate the process, the formulation of the CQDs solution commenced via a hydrothermal treatment applied to the WRN, adhering to the methodology outlined in our earlier research [44,45]. In the standard synthesis protocol, 100 g of WRN underwent thorough grinding to yield a consistent paste using a mortar. Subsequently, this paste was combined with 200 g of deionized water. The ensuing mixture underwent heating within a 500 mL Teflon-lined autoclave, where it was subjected to a temperature of 200 °C for a duration of 10 h. A brown CQDs solution, accompanied by black-gray sediment, was successfully acquired. Employing vacuum filtration, the brown CQDs solution was isolated, intended for subsequent fabrication of CQDs/FeOx photocatalytic composites, while the solid portion was separated to yield hydrothermal carbon (HTC) powder, which could be further treated to produce activated carbon [44]. Simultaneously, the IOS powder underwent calcination at various temperatures (100 °C, 200 °C, 300 °C, 400 °C, 500 °C) in an air atmosphere using a SGM6812CK tube furnace (Sigma, Luoyang, China) for 4 h, producing FeOx as a brown powder.
Next, a volume of 40 mL from the CQDs solution was skillfully combined with an appropriate quantity of FeOx, choosing from three different amounts (0.25 g, 0.5 g, 1 g), in precisely measured proportions. Through meticulous magnetic stirring at room temperature for a duration of 0.5 h, a consistent and homogenous suspension was created. Subsequently, this well-mixed composition was placed into a 50 mL Teflon-lined autoclave, undergoing heating at a temperature of 85 °C for a span of 3 h to facilitate the formation of the composite structure. Following this process, the composite material was retrieved and subjected to centrifugation, followed by thorough triple washing with distilled water. Upon being subjected to vacuum drying at 60 °C, the resulting product emerged as the CQDs/FeOx photocatalytic composite material, taking the form of a deep brown powder. The samples were labeled as CQDs/FeOx–1 to CQDs/FeOx–7 based on the calcination temperature of the IOS and the amount of FeOx used. The relevant formulation design and elemental composition are presented in Table 1.
Table 1.
Formulation design of CQDs/FeOx composites.
| Serial Number | Calcination Temperature of IOS (℃) | Dosage of FeOx (g) | Elemental Composition (wt%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | O | C | Mn | Al | Si | Ca | |||
| CQDs/FeOx–1 | 100 | 0.5 | 63.17 | 24.36 | 11.23 | 0.19 | 0.41 | 0.35 | 0.29 |
| CQDs/FeOx–2 | 200 | 0.5 | 62.75 | 24.66 | 11.34 | 0.2 | 0.42 | 0.36 | 0.27 |
| CQDs/FeOx–3 | 300 | 0.5 | 62.6 | 24.94 | 11.14 | 0.21 | 0.44 | 0.35 | 0.32 |
| CQDs/FeOx–4 | 400 | 0.5 | 62.91 | 24.87 | 10.89 | 0.23 | 0.44 | 0.34 | 0.32 |
| CQDs/FeOx–5 | 500 | 0.5 | 62.95 | 24.58 | 11.24 | 0.22 | 0.39 | 0.33 | 0.29 |
| CQDs/FeOx–6 | 300 | 0.25 | 56.24 | 22.38 | 20.21 | 0.19 | 0.4 | 0.32 | 0.26 |
| CQDs/FeOx–7 | 300 | 1 | 65.88 | 26.2 | 6.56 | 0.22 | 0.46 | 0.35 | 0.33 |
2.3. General Characterization and Measurement of Photocatalytic Performance
The characterizations and photocatalytic degradation experiments of the CQDs/FeOx composite material were conducted following the methods outlined in our previous reports [44,45]. For in-depth details, please refer to Supplementary Materials S1 and S2 in the ESI.
3. Result and Discussion
3.1. Structural Characterization
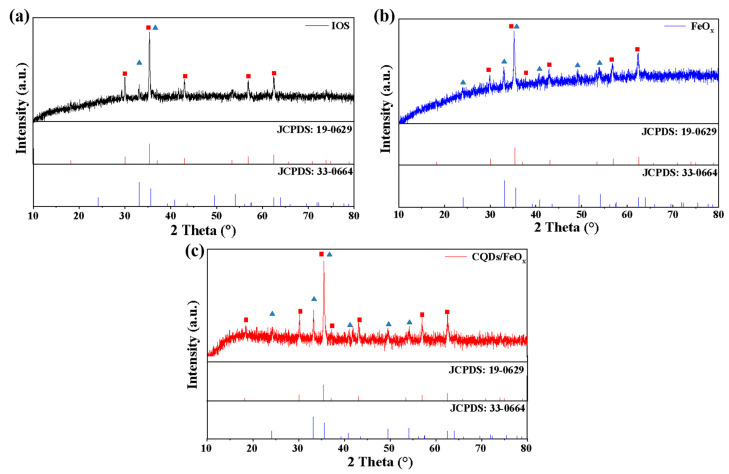
The PXRD analysis of the CQDs/FeOx composite (CQDs/FeOx–3 sample), FeOx powder (achieved through the calcination of IOS at 300 °C), and IOS (raw material) is depicted in Figure 2. Evidently, the waste IOS contained two main phases. Among them, the characteristic peaks at 30.0°, 35.4°, 37.0°, 43.1°, 56.9°, and 62.5° corresponded to the (220), (311), (222), (400), (511), and (440) crystal planes, respectively, which could be attributed to magnetite (Fe3O4, JCPDS card no. 19-0629). Additionally, the characteristic peaks at 33.1° and 35.6° could be attributed to the (104) and (110) crystal planes of hematite (α-Fe2O3, JCPDS card no. 33-0664). This indicated that the IOS mainly contained Fe3O4 and α-Fe2O3, with Fe3O4 being dominant. Combining this with the results of the elemental composition analysis (See Table 2), the Fe/O mass ratio in the IOS was 2.59, and it could be calculated that there was only about 10.8% of α-Fe2O3 in the mixed phase of iron oxide. Typically, the band gap of magnetite (Fe3O4) was too small to exhibit an effective photocatalytic ability. As a result, the iron oxide photocatalytic component was mainly α-Fe2O3. Therefore, it was foreseeable that the waste IOS could not be directly used as a photocatalytic material or directly combined with CQDs due to the low content of α-Fe2O3, making calcination treatment necessary to enhance its photocatalytic potential.
Figure 2.
PXRD pattern of IOS (a), FeOx (b) and CQDs/FeOx composite (c). The red square and blue triangle represent the diffraction peaks of Fe3O4 and α-Fe2O3, respectively.
Table 2.
Comparison of composition element content of CQDs/FeOx composite, FeOx and IOS.
| Serial Number | Elemental Composition (wt%) | ||||||
|---|---|---|---|---|---|---|---|
| Fe | O | C | Al | Si | Ca | Mn | |
| IOS | 71.09 | 27.41 | - | 0.23 | 0.47 | 0.42 | 0.38 |
| FeOx a | 70.46 | 28.05 | - | 0.21 | 0.49 | 0.4 | 0.39 |
| CQDs/FeOx composite b | 62.6 | 24.94 | 11.14 | 0.21 | 0.44 | 0.35 | 0.32 |
a calcining IOS at 300 °C in an air atmosphere for 4 h; b CQDs/FeOx–3 sample.
Comparing the PXRD pattern of the FeOx powders obtained through the calcination of IOS at 300 ℃ with that of IOS, it was evident that the diffraction peaks of the α-Fe2O3 were significantly enhanced after calcination. The characteristic peaks at 24.1°, 33.1°, 35.6°, 40.9°, 49.5°, and 54.1° corresponded to the (012), (104), (110), (113), (024), and (116) crystal planes of α-Fe2O3, respectively. Additionally, based on compositional analysis, the Fe/O mass ratio decreased to 2.51, indicating that the FeOx powder contained 43.3% of α-Fe2O3. This transformation implied that calcination in air could convert a portion of the initially non-photocatalytic Fe3O4 into photocatalytically active α-Fe2O3, which laid the foundation for obtaining practical photocatalytic materials after the composite with CQDs. The PXRD patterns of the CQDs/FeOx composite and FeOx exhibited resemblances; however, the diffraction peaks corresponding to the CQDs within the composite were not prominently discernible due to their relatively modest concentration.
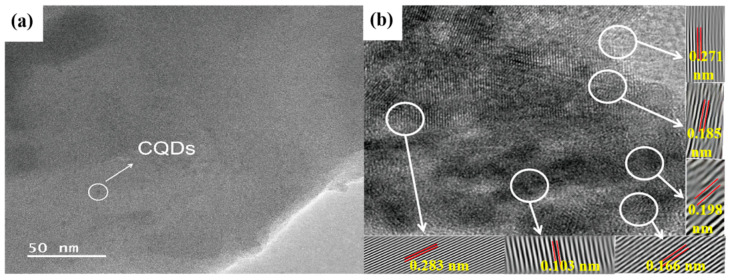
The results of the particle size distribution testing indicated that the obtained CQDs/FeOx composite (CQDs/FeOx–3 sample) exhibited a Z-average particle size value of 127.8 ± 63.26 nm (refer to Figure S1 in ESI). The outcomes of the TEM analysis for the CQDs/FeOx composite (CQDs/FeOx–3 sample) are visually depicted in Figure 3a. It could be observed that the CQDs/FeOx composites exhibited an irregular lamellar structure, with uniformly dispersed spherical CQDs particles on the FeOx surface. In Figure 3b, the HRTEM image vividly revealed the intricate lattice arrangement of the FeOx and CQDs. The lattice stripes, exhibiting spacings of 0.185 nm, 0.198 nm, and 0.271 nm, corresponded, respectively, to the (104), (110), and (024) crystal planes of α-Fe2O3. Furthermore, discernible lattice stripes with spacings of 0.103 nm and 0.168 nm aligned with the (111) and (220) crystal planes of Fe3O4. These observations indicated that the obtained FeOx was primarily a mixture of magnetite (Fe3O4) and hematite (α-Fe2O3), which aligned with the PXRD results. Moreover, crystalline planes with a lattice spacing of about 0.283 nm, corresponding to the (020) crystalline planes of CQDs [50,51], were also observed, confirming the successful combination of the WRN-based CQDs and FeOx in the resulting composite.
Figure 3.
The TEM image (a) and HRTEM image (b) of CQDs/FeOx composite based on WRN and IOS.
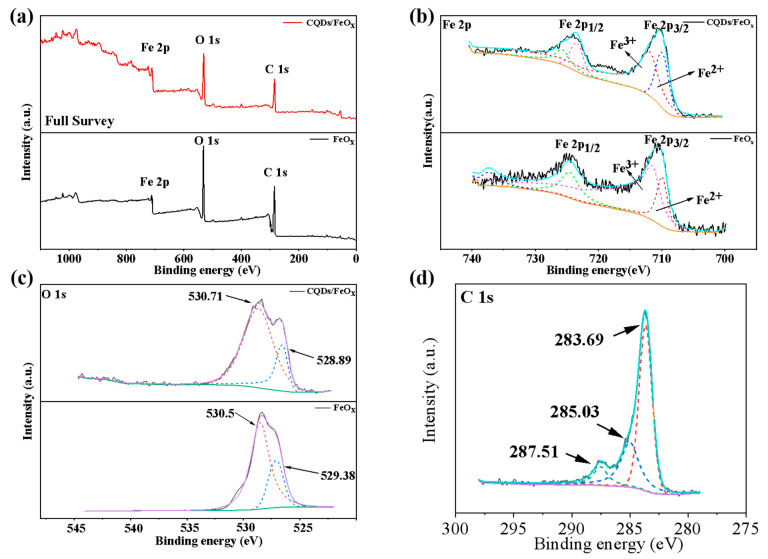
The XPS spectra of both the CQDs/FeOx composite and FeOx powders are displayed in Figure 4 and summarized in Table 3. Figure 4a highlights the elemental presence of Fe, O, and C within the CQDs/FeOx composite. Notably, Figure 4b and Table 2 elucidate that the Fe 2p spectra disclosed characteristic peaks at 724.67 and 710.49 eV, corresponding to the Fe(2p1/2) and Fe(2p3/2) signals, respectively. A noteworthy separation of divalent and trivalent iron signals was discernible within the Fe(2p3/2) signal, indicative of the presence of both Fe2+ and Fe3+ ions in the resulting CQDs/FeOx composite. In the high-resolution spectrum of O 1s, the CQDs/FeOx complex displayed distinct Fe–O and C–O bonds, hosting characteristic signals positioned at 530.71 and 528.29 eV, respectively. In contrast, the O 1s spectrum of the FeOx calcined at 300 °C displayed two distinct peaks at 530.5 and 529.38 eV, attributing them to the Fe–O bond and the hydroxyl group present on the FeOx surface, respectively. This led to the inference that the interaction between CQDs and FeOx involved the carboxyl group within the CQDs and the Fe–OH group on the surface of the FeOx, leading to the vanishing of the surface hydroxyl signal and the emergence of the C–O bond signal, as indicated in Figure 4c and Table 3. Additionally, in the high-resolution C 1s spectrum of the CQDs/FeOx composite, the peak at 283.69 eV could be attributed to the C–C bond within the CQDs. Simultaneously, the signals centered at 285.03 and 287.51 eV corresponded to the C–O and C=C bonds of CQDs, respectively, confirming the successful integration of the CQDs and FeOx within the resultant composites.
Figure 4.
The full XPS (a), as well as Fe 2p ((b), black: original data, blue: overall fitting curve, green: Fe3+ curve at Fe 2p1/2, purple: Fe2+ curve at Fe 2p1/2, red: Fe3+ curve at Fe 2p3/2, navy blue: Fe2+ curve at Fe 2p3/2, orange curve: bottom line), O 1s ((c), black: original data, purple: overall fitting curve, red and blue: peak fitting, green: bottom line) and C 1s ((d), CQDs/FeOx composite only, black: original data, blue: overall fitting curve, red and green: peak fitting, purple: bottom line) high-resolution spectrum of FeOx powder and CQDs/FeOx composite.
Table 3.
XPS peak distribution of FeOx powder and CQDs/ FeOx composite based on waste rice noodle.
| Photocatalyst | Element | Peak (eV) | Surface Group | Assignment |
|---|---|---|---|---|
| CQDs/FeOx composite a | C 1s | 283.69 | C | Graphitic carbon |
| 285.03 | C–O | Alcoholic or etheric structure in CQDs | ||
| 287.51 | C=C | Aromatic ring of CQDs | ||
| O 1s | 530.71 | Fe–O | Oxygen bonded to iron | |
| 528.29 | C–O | Oxygen singly bonded to CQDs | ||
| Fe 2p | 710.49 | Fe | Fe (2p3/2) | |
| 724.67 | Fe | Fe (2p1/2) | ||
| FeOx b | O 1s | 530.5 | Fe–O | Oxygen bonded to iron |
| 529.38 | Fe–OH | Surface hydroxyl group of FeOx | ||
| Fe 2p | 710.35 | Fe | Fe (2p3/2) | |
| 724.71 | Fe | Fe (2p1/2) |
a CQDs/FeOx–3 sample; b calcining IOS at 300 °C in an air atmosphere for 4 h.
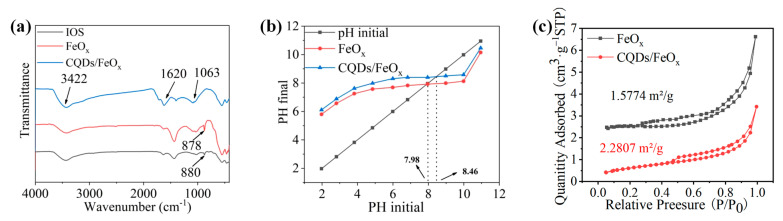
As shown in Figure 5a, without the combination of CQDs, the IOS and FeOx exhibited an obvious Fe–OH stretching vibration peak at around 880 cm−1 in their IR spectra. However, this peak disappeared after the composite of FeOx with CQDs. Paired with the XPS findings, it is conceivable that the intricate interplay between the CQDs and FeOx constituted a response involving the carboxyl group within the CQDs and the hydroxyl group located on the FeOx surface. Furthermore, characteristic absorption peaks of CQDs were observed in the IR spectra of the CQDs/FeOx composites, including the stretching vibration (3422 cm−1) and bending vibration (1620 cm−1) of the O–H bond on the CQDs, and the stretching vibration (1063 cm−1) of the C–O bond, etc. In addition, due to the presence of organic groups such as hydroxyl and carboxyl groups in the CQDs, the PZC value of the CQDs/FeOx composites (8.46) was higher than that of the non-composite FeOx powder (7.98) (See Figure 5b). In addition, due to larger feedstock particles, the CQDs/FeOx composite exhibited a relatively low specific surface area (2.28 m2 g−1) compared with the CQDs/TiO2 [44] and CQDs/ZnO [45] photocatalytic composites in our previous report; however, it was still slightly higher than that of the non-composite FeOx powder (1.57 m2 g−1) (See Figure 5c).
Figure 5.
(a) IR spectra of IOS, FeOx powder and CQDs/FeOx composite; (b,c) The PZC value (b) and BET (c) of FeOx powder and CQDs/FeOx composite.
3.2. Photocatalytic Performance of CQDs/FeOx Composites
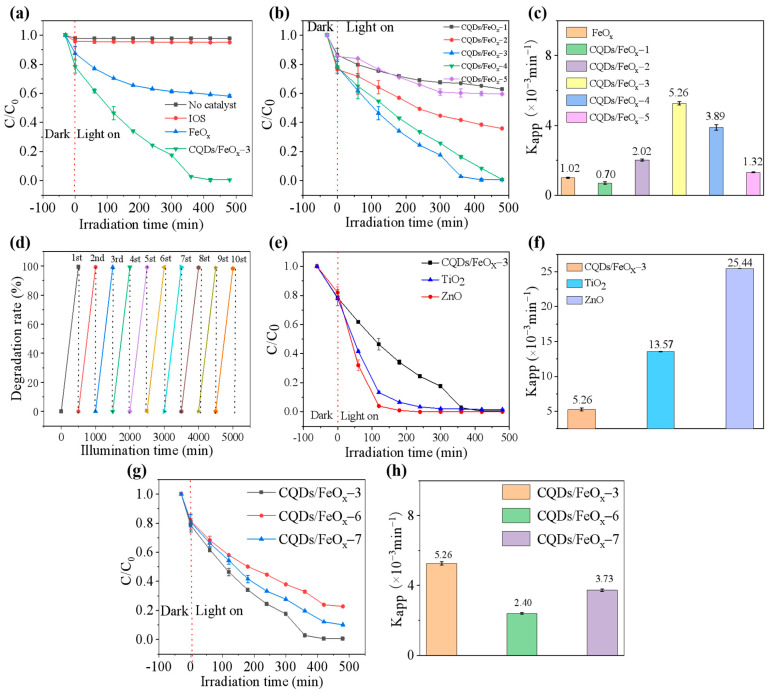
The resulting CQDs/FeOx composite exhibited excellent photocatalytic degradation efficiency towards various organic pollutants. Figure 6a underscores the formidable challenge of degrading methylene blue, a prevalent and highly toxic pollutant in dyeing wastewater, using 405 nm visible purple light. In the absence of a catalyst, its degradation rate remained at a mere 2.3%, even after an extended duration of 8 h (480 min). Due to its non-photocatalytic Fe3O4 component, the IOS itself lacked the ability for photocatalytic degradation to methylene blue, as its degradation rate was only 4.9% after 480 min of light irradiation, which was almost indistinguishable from the degradation without the catalyst. However, after calcination at 300 °C, a portion of the Fe3O4 in the IOS was converted into α-Fe2O3, resulting in the FeOx with a certain photocatalytic degradation ability. The degradation rate of the FeOx towards methylene blue reached 41.8% after 480 min of light irradiation, with an apparent degradation rate constant of 1.03 × 10−3 min−1. However, complete degradation could not be achieved under these conditions. In contrast, the catalytic effect of the CQDs/FeOx composite (CQDs/FeOx–3 sample) on methylene blue significantly improved after incorporating the WRN-based CQDs into the FeOx. The composite demonstrated a good photocatalytic degradation rate (up to 99.30% within 480 min) and a relatively high degradation rate constant (5.26 × 10−3 min−1), enabling the complete degradation of methylene blue (See Figure S2 in ESI).
Figure 6.
(a) Photocatalytic degradation rates of IOS, FeOx powder and CQDs/FeOx composites (CQDs/FeOx–3 sample) at 405 nm purple light for methylene blue at different irradiation times; (b) Photocatalytic degradation rates of CQDs/FeOx composites prepared at different calcination temperatures for methylene blue at 405 nm purple light at different irradiation times; (c) Apparent degradation rate constants (Kapp) of CQDs/FeOx composites and FeOx powder prepared at different calcination temperatures; (d) Photocatalytic degradation performance of CQDs/FeOx composites across varying photocatalytic cycles employing a 500-min operational cycle; (e,f) The photocatalytic degradation rates (e) and apparent degradation rate constant (Kapp, (f)) of CQDs/FeOx–3 sample, commercial TiO2 and ZnO; (g,h) Photocatalytic degradation rates (g) and apparent degradation rate constant (Kapp, (h)) of CQDs/FeOx composites with different carbon contents.
It was found that achieving a good photocatalytic performance for the CQDs/FeOx composite required the appropriate calcination of the IOS in an air atmosphere to enhance the content of α-Fe2O3. The degradation rates of methylene blue for CQDs/FeOx composite materials obtained at different calcination temperatures (samples CQDs/FeOx–1 to CQDs/FeOx–5) are shown in Figure 6b,c. Combined with the results of the phase and elemental composition, it was observed that without proper high-temperature calcination to increase the content of α-Fe2O3, even with the incorporation of CQDs, the catalytic effect could hardly be improved. For instance, the CQDs/FeOx–1 sample obtained by heating the IOS at 100 °C had a Fe/O mass ratio of 2.59 due to the relatively low calcination temperature. It was found that only about 10.9% of α-Fe2O3 was present in the mixed FeOx phase, similar to the IOS as a raw material. Consequently, despite containing CQDs, the photocatalytic degradation of CQDs/FeOx–1 remained poor. After 480 min of light exposure, its degradation rate to methylene blue was only 35.79%, and the degradation rate constant was merely 7.04 × 10−4 min−1, which is even worse than the FeOx sample without CQDs but calcined at 300 °C. There are reports indicating that, when heated in air at an appropriate temperature (around 300 °C), Fe3O4 exhibits a tendency to transform into α-Fe2O3 [52,53,54]. As shown in Table 1, with enhancing the calcination temperature, the Fe/O mass ratio of the composite FeOx material showed a trend of initially decreasing and then increasing, indicating that the content of α-Fe2O3 first increased and then decreased. Among them, the CQDs/FeOx–3 sample prepared with IOS calcined at 300 °C had the smallest Fe/O mass ratio (2.51), which corresponded to the highest α-Fe2O3 content (44%). Therefore, it exhibited the best photocatalytic performance and achieved the complete degradation of methylene blue within 480 min, with a degradation rate constant of 5.26 × 10−3 min−1. Furthermore, the material demonstrated excellent recyclability, and its degradation rate remained above 98% even after ten cycles of photocatalysis (See Figure 6d). However, when compared to commercially available nanoscale TiO2 or ZnO, the photocatalytic degradation efficiency of the obtained CQDs/FeOx photocatalytic material was relatively low (See Figure 6e,f). Nevertheless, due to its complete reliance on waste materials as feedstock, the cost of the CQDs/FeOx photocatalytic material is significantly lower. Additionally, it possesses strong magnetism, unlike commercial TiO2 or ZnO, enabling convenient recovery. Therefore, it may present a more competitive option for large-scale water purification.
Additionally, the loading amount of CQDs also affects the photocatalytic degradation capability of the CQDs/FeOx composite material. As shown in Figure 6g,h, at lower carbon contents, the photocatalytic degradation efficiency of the CQDs/FeOx composite material improved with the increasing carbon content. The CQDs/FeOx composite material with a carbon content of approximately 11 wt% (CQDs/FeOx–3 sample) exhibited the best photocatalytic performance, with a degradation rate constant of 5.26 × 10−3 min−1 for methylene blue. However, it was found that further increasing the loading amount of CQDs may have an adverse effect on the photocatalytic performance. For example, in the case of the CQDs/FeOx–7 sample featuring a carbon content of 20.2 wt%, a comparatively reduced degradation rate constant of 3.73 × 10−3 min−1 was observed for methylene blue degradation. This phenomenon could be attributed to the potential shielding influence stemming from the presence of carbon-based constituents [55].
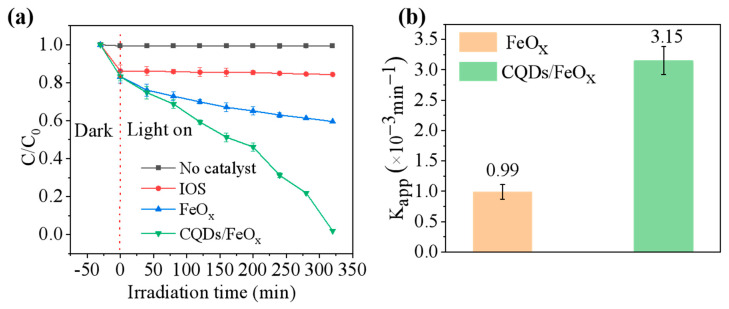
The obtained CQDs/FeOx composite material could also be utilized for controlling antibiotic residues, as shown in Figure 7. Under 405 nm purple light, the CQDs/FeOx composite (CQDs/FeOx–3 sample) could degrade 98.21% of tetracycline within 320 min, with an apparent degradation rate constant of 3.73 × 10−3 min−1. In comparison, under the same conditions, the FeOx powder without CQDs could only degrade 40.45% of tetracycline, with an apparent degradation rate constant of 1.29 × 10−3 min−1.
Figure 7.
(a) Photocatalytic degradation rates of IOS, FeOx powder and CQDs/FeOx composite (CQDs/FeOx–3 sample) at 405 nm purple light for tetracycline hydrochloride at different irradiation times; (b) Apparent degradation rate constants (Kapp) of CQDs/FeOx composite (CQDs/FeOx–3 sample) and FeOx powder.
Compared with other CQDs/iron oxide composites using different iron and carbon sources (Table 4), the CQDs/FeOx composite reported in this paper demonstrated a comparably good photocatalytic performance. Moreover, due to the complete utilization of waste materials (IOS and WRN) as raw sources, the synthesis cost of this material was significantly reduced compared to other CQDs/iron oxide composites [29,30,31,32,33,34]. This complete conversion of waste to treasure makes it more environmentally friendly and exhibits outstanding sustainability, making it more suitable for large-scale, industrial applications in water purification projects. In comparison to our previous reports on CQDs/TiO2 and CQDs/ZnO composites based on WRN, the CQDs/FeOx composite exhibited a weaker photocatalytic performance (See Table 5). However, due to the presence of magnetic Fe3O4 components, it offers the advantage of simplified recovery procedures while ensuring high recyclability. Additionally, as the oxide component is derived from waste IOS, it eliminates the need for commercial reagents, further reducing the costs and promoting environmental friendliness.
Table 4.
Photocatalytic degradation performance of CQDs/iron oxide composites utilizing various iron and carbon sources.
| Iron Source | Carbon Source of CQDs | Light Source | Pollutant | Pollutant Concentration | Photocatalyst Dosage (g/L) | Irradiation Times (min) | Degradation Rate (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Waste IOS | WRN | Purple light lamp (20 W, wavelength: 405 nm) | methylene blue | 20 mg/L | 2 | 480 | 99.30 | This work |
| tetracycline | 20 mg/L | 2 | 320 | 98.21 | ||||
| Commercially-available magnietic Fe3O4 nanoparticles | Glucose | Xe lamp (400 W, wavelength > 420 nm) | methylene blue (in NaOH solution) | 1 × 10–3 mol/L | 1 | 30 | 83 | [29] |
| Commercial γ-Fe2O3 | Glucose | Xe lamp (300 W, wavelength: 455 nm) | sulfamethoxazole (SMX) | 10 mg/L | 0.2 | 120 | 95 | [30] |
| FeSO4·7H2O | Citric acid | Xe lamp with a 420 nm cutoff filter (350 W) | tetracycline, (0.50 mM of H2O2 was added) | 20 mg/L | 0.25 | 60 | 93 | [31] |
| Fe(NO3)3·9H2O | Citric acid | HPMVL visible light lamp (250 W) | Oxytetracycline | 10 mg/L | 0.2 | 100 | 98 | [32] |
| FeSO4·7H2O | Citric acid | Xe lamp (300 W, wavelength > 420 nm) | Metronidazole | 30 mg/L | 0.2 | 45 | 99.36 | [33] |
| FeCl3·6H2O | Citric acid and Ethylenediamine | Xe lamp (300 W, wavelength: 420 nm) | Methylene blue | 20 mg/L | 0.5 | 120 | 99.3 | [34] |
Table 5.
Photocatalytic degradation performance of different CQDs/metal oxide composites based on WRN.
| Metal Source | Carbon Source of CQDs | Light Source | Pollutant | Pollutant Concentration | Photocatalyst Dosage (g/L) | Irradiation Times (min) | Degradation Rate (%) | Reference |
|---|---|---|---|---|---|---|---|---|
| Waste IOS | WRN | Purple light lamp (20 W, wavelength: 405 nm) | methylene blue | 20 mg/L | 2 | 480 | 99.30 | This work |
| tetracycline | 20 mg/L | 2 | 320 | 98.21 | ||||
| Commercial TiO2 | WRN | Purple light lamp (20 W, wavelength: 405 nm) | methylene blue | 20 mg/L | 4 | 80 | 99.87 | [44] |
| Commercial ZnO | WRN | Purple light lamp (20 W, wavelength: 405 nm) | methylene blue | 20 mg/L | 2 | 10 | 98.88 | [45] |
| tetracycline | 20 mg/L | 2 | 10 | 98.21 |
3.3. Magnetic Properties of CQDs/FeOx Composites
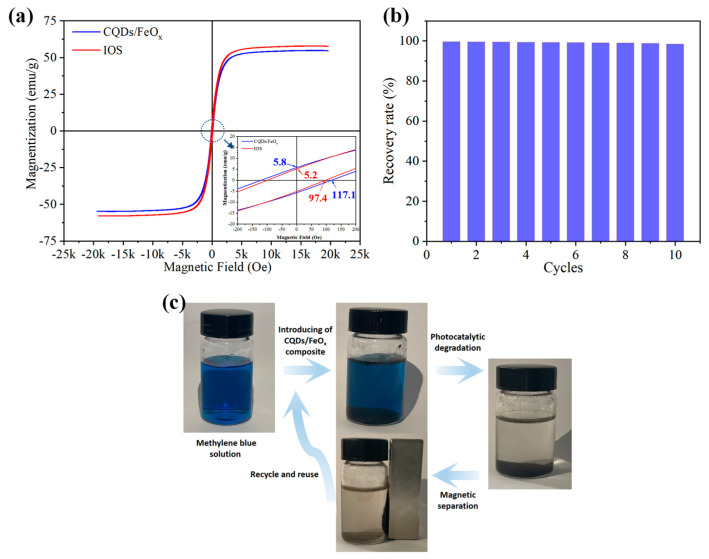
In addition to exhibiting efficient photocatalytic degradation to various organic pollutants, the resulting CQDs/FeOx composite also demonstrated outstanding magnetic properties. The magnetization curves of the CQDs/FeOx composite and the raw IOS were tested and are shown in Figure 8a. The IOS powder exhibited remarkable soft ferromagnetism at room temperature, characterized by a low coercivity of 97.4 Oe, a low residual magnetization intensity of 5.2 emu/g, and a high saturation magnetization intensity of 5.8 emu/g, primarily attributed to the abundance of Fe3O4 particles in the material.
Figure 8.
(a) Magnetization curves of IOS and CQDs/FeOx composite (CQDs/FeOx–3 sample); (b) Mass recovery rate of CQDs/FeOx composite (CQDs/FeOx–3 sample) across varying photocatalytic cycles; (c) Schematic illustration of the recycle and reuse of CQDs/FeOx composite using magnetic separation.
As mentioned earlier, after the process of calcination and compounding, the magnetic properties of the CQDs/FeOx composites slightly weakened due to the reduced Fe3O4 content. Nevertheless, its saturation magnetization intensity remained at approximately 54.6 emu/g, along with low coercivity (117.1 Oe) and residual magnetization intensity (5.8 emu/g). Therefore, the CQDs/FeOx composite still exhibits exceptional soft magnetic characteristics, making it a promising candidate for various magnetic applications.
As depicted in Figure 8b,c, the magnetic properties of the CQDs/FeOx composite material enable efficient and convenient recyclability in photocatalytic applications. Following the photocatalytic process, the CQDs/FeOx composite can be effortlessly separated using a magnetic field, achieving a mass recovery rate of 99.62% after the initial photocatalytic cycle and 98.45% after ten cycles of photocatalysis. Additionally, iron oxide photocatalysts have demonstrated excellent environmental compatibility, with minimal toxicity to fish and algae [56,57,58]. Consequently, the resulting CQDs/FeOx photocatalyst holds significant promise for large-scale industrial water purification.
3.4. Photocatalytic Mechanism of CQDs/FeOx Composites
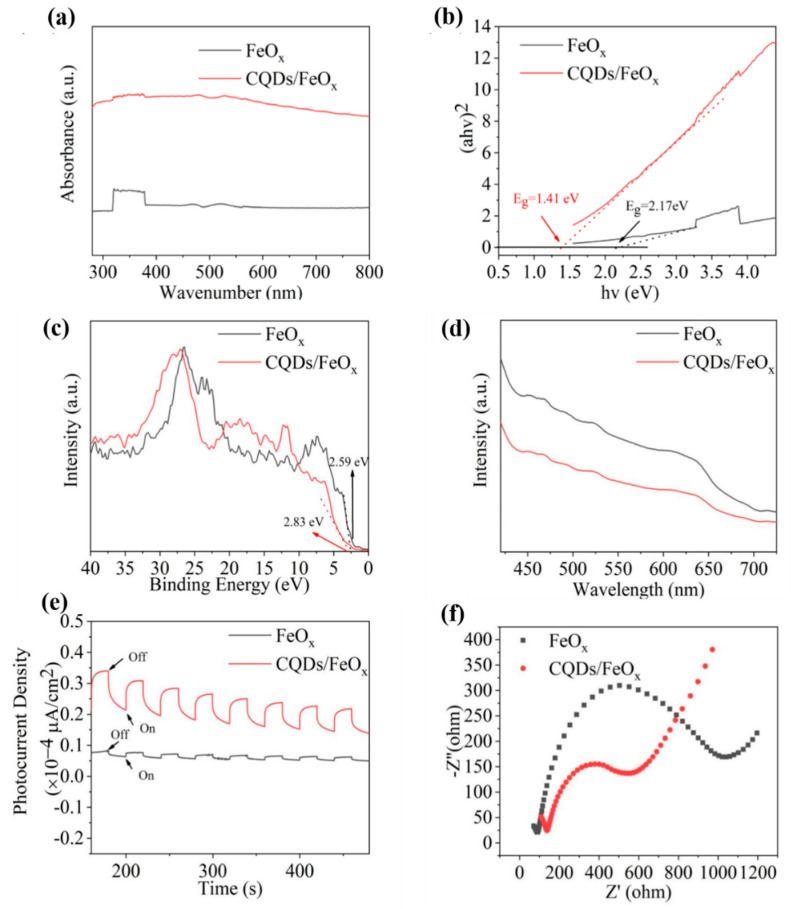
To delve deeper into the photocatalytic mechanism of the resulting CQDs/FeOx composite, the UV-VIS diffuse reflectance spectra of both the FeOx and the CQDs/FeOx composite are illustrated in Figure 9a. Evidently, even after calcination at 300 °C, the main energy absorption region of the FeOx inorganic phase was in the ultraviolet region, with almost no absorption in the visible wavelength range above 380 nm. In contrast, the CQDs/FeOx composite material exhibited strong absorption throughout the entire visible light region, indicating that the introduction of CQDs enabled the composite material to utilize the energy in the visible light region more effectively, thereby generating more electron-hole pairs. Moreover, the determination of band gaps for the FeOx and CQDs/FeOx samples was performed through the application of the Tauc plot method [59,60], as depicted in Figure 9b. The band gap of the FeOx was 2.17 eV, but after introducing CQDs, the band gap of the CQDs/FeOx composite material was further reduced to 1.40 eV. This narrower band gap allowed for more the effective utilization of energy in the visible light region, significantly promoting electron transitions and enhancing the photocatalytic degradation performance.
Figure 9.
The UV-vis absorption spectra (a), Tauc plot curves (b), VB XPS spectra (c), PL spectra (d), PCR (e) and EIS (f) of the CQDs/FeOx composite and FeOx.
The valence band (VB) potentials of the CQDs/FeOx composite and FeOx powder are determined using XPS valence spectra. As shown in Figure 9c, due to the presence of Fe2O3, the VB potential of the FeOx powder was 2.59 eV, which was more positive than E0(·OH, H+/H2O) (2.38 eV vs. NHE). This suggested that the FeOx powder could oxidize water to generate hydroxyl radical (·OH). However, the conduction band (CB) potential (ECB = EVB − Eg) of the FeOx was determined to be 0.42 eV, which was more positive than the standard electrode potential E0(O2, H+/·O2H) for superoxide radicals (−0.046 eV vs. NHE). This indicated that the FeOx powder could not reduce oxygen in water to produce superoxide radical (O2·–). On the other hand, the introduction of CQDs raised the VB potential of the CQDs/FeOx composite material to 2.83 eV, enabling better oxidation of water to form photocatalytically active hydroxyl radical. Combining its own band gap (1.40 eV) and the band gap of CQDs (2.14 eV), the CB potential was calculated to be −0.08 eV, which was lower than E0(O2, H+/·O2H) for superoxide radicals (−0.046 eV vs. NHE). This indicated that the CQDs/FeOx composite material could reduce oxygen in water to generate photocatalytically active superoxide radical.
The photoluminescence emission profiles of both the CQDs/FeOx composite material and FeOx are presented in Figure 9d. The fluorescence emission intensity exhibited by the CQDs/FeOx composite material was notably subdued in comparison to that of the FeOx. This observation implies that the incorporation of CQDs could proficiently curtail the recombination of photogenerated electron-hole pairs, thereby significantly contributing to the enhancement of the photocatalytic degradation efficacy. The enhanced mechanism of the CQDs/FeOx composite material on the catalytic performance of the FeOx was also demonstrated through transient photocurrent response (PCR) under visible light irradiation and electrochemical impedance spectra (EIS). As shown in Figure 9e, under purple light illumination, the photocurrent intensity of the CQDs/FeOx composite material was approximately 11 times that of the FeOx without CQDs, indicating that the CQDs/FeOx composite could achieve a more efficient interface charge transfer and more effective electron-hole pair separation. The lower probability of photogenerated electron-hole recombination resulted in a significant improvement in the photocatalytic degradation performance. Additionally, the Nyquist plot of the CQDs/FeOx composite displayed a smaller semicircle diameter than that of the FeOx powder, indicating that the resulting CQDs/FeOx composite exhibited lower charge transfer resistance than the FeOx powder. This ensured a more efficient interface charge transfer and more effective electron-hole pair separation, consistent with the photocurrent analysis results (see Figure 9f).
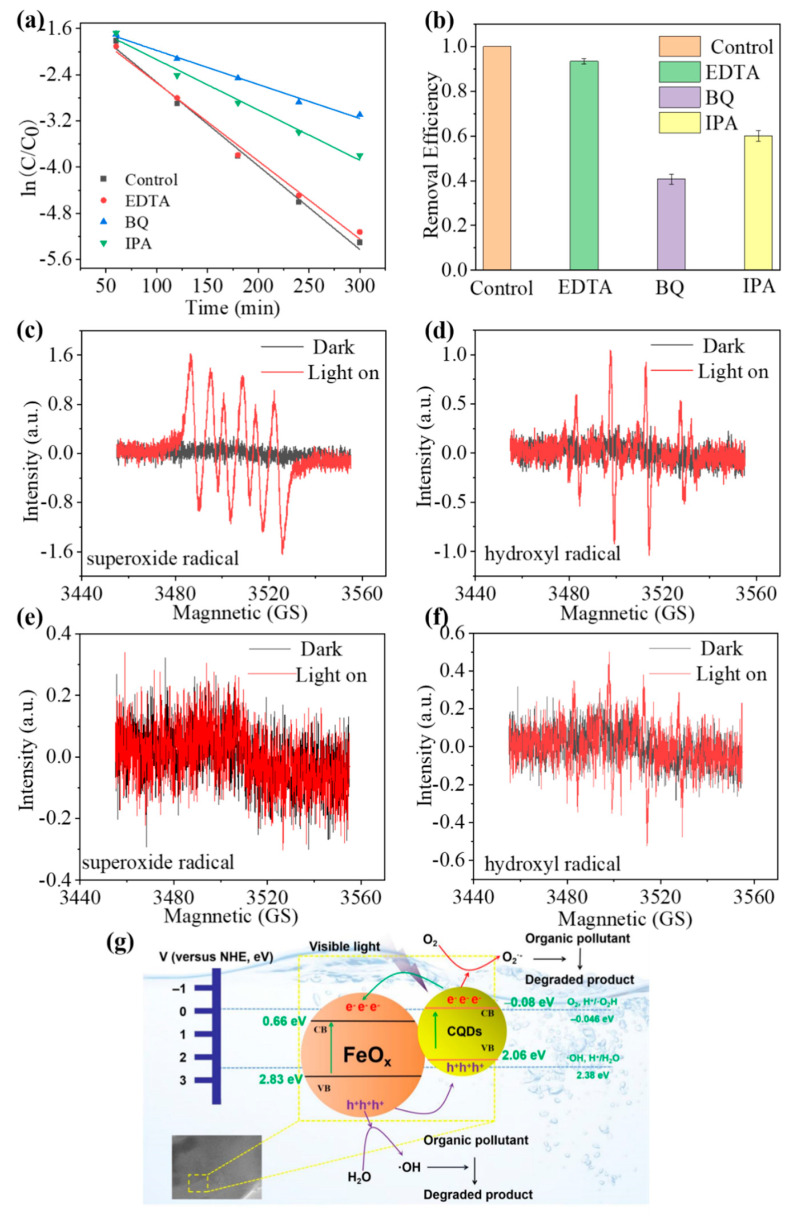
The impacts of distinct quenching agents (EDTA–2Na, BQ and IPA) on the photodegradation process of methylene blue are elucidated in Figure 10a,b. Notably, the introduction of EDTA–2Na marginally curtailed the photocatalytic degradation efficacy of the CQDs/FeOx composite material, yielding a photocatalytic efficiency of 93.44% in comparison to the absence of quenching agents. This observation underscores that photogenerated holes (h+) played a minor role and were not the primary drivers of the photocatalytic activity. In contrast, the incorporation of BQ or IPA substantially impeded the degradation efficiency, yielding photocatalytic efficiencies of 40.75% and 60.15%, respectively, as opposed to the scenario without quenching agents. This phenomenon implies that both superoxide radicals (O2·–) and hydroxyl radicals (·OH) stood as the predominant active species in the photocatalytic degradation mechanism, with O2·– playing a more pronounced role. Furthermore, using DMPO as a radical trapping agent, electron spin resonance spectroscopy (ESR) was carried out to study the active oxygen species generated by the CQDs/FeOx composite and FeOx. As shown in Figure 10c–f, the addition of the CQDs/FeOx composite resulted in strong characteristic peaks of both superoxide radical (O2·–) and hydroxyl radical (·OH). This indicates that the CQDs/FeOx composite material could reduce adsorbed O2 to form superoxide radical (O2·–) and oxidize adsorbed H2O to form hydroxyl radical (·OH) under light irradiation. In contrast, under the same test conditions, the FeOx powder could not generate superoxide radical (O2·–) effectively, while its signal of hydroxyl radicals was weak.
Figure 10.
(a,b) Impact of various quenching agents (EDTA–2Na, BQ or IBA) on the photodegradation of methylene blue under 405 nm purple light. (c,d) ESR spectra of the CQDs/FeOx composite in methanol (c) and water (d) using DMPO as a radical trapping agent. (e,f) ESR spectra of FeOx in methanol (e) and water (f) using DMPO as a radical trapping agent. (g) Diagram illustrating the photocatalytic mechanism of the CQDs/FeOx composite.
As shown in Figure 10g, the possible photocatalytic mechanism of the CQDs/FeOx composite material was similar to that of the CQDs/TiO2 [44] and CQDs/ZnO [45] composites in our previous report. Upon exposure to visible light, the CQDs/FeOx composite undergoes a dynamic process: the CQDs become readily excited by photogenerated electrons situated in the conduction band (CB), leaving behind holes in the valence band (VB). This excitation triggers rapid spatial electron transfer between the CQDs and FeOx particles, effectively suppressing recombination and yielding the enhanced separation of electron-hole pairs. As a result, photogenerated electrons amass in the CB of the CQDs, while the holes populate the VB of FeOx, with each entity primed for their distinct roles in photocatalytic reactions. Photogenerated holes engage with H2O to yield a profusion of ·OH radicals, while photogenerated electrons react with O2, leading to an abundant production of O2·– radicals. These generated O2·– and ·OH radicals collectively orchestrate the degradation of diverse organic pollutants, thereby showcasing exceptional prowess in the realm of photocatalytic degradation activity.
4. Conclusions
In this study, we successfully synthesized low-cost CQDs/FeOx composites by combining waste rice noodle (WRN) and iron oxide scale (IOS) and assessed their photocatalytic performance. The key steps in our synthesis strategy were identified: first, calcining IOS in an air atmosphere to enhance the photocatalytically active α-Fe2O3 content; second, incorporating WRN-based CQDs into FeOx to enhance the electron-hole pair separation, leading to increased O2 reduction and H2O oxidation. This significantly improved the photocatalytic performance. The resulting CQDs/FeOx composites efficiently degraded various organic pollutants under purple light irradiation and had superior magnetic properties, allowing for easy separation and reuse.
Our CQDs/FeOx composite achieved the 100% conversion of WRN and IOS waste into high-value functional materials without additional synthetic additives. Compared to other reported photocatalytic composites, such as the CQDs/TiO2 and CQDs/ZnO from our previous work and other CQDs/iron oxide composites using synthetic reagents, our CQDs/FeOx composite is more cost-effective, environmentally friendly, and sustainable. Its magnetic properties enable rapid separation and efficient reuse, making it a promising candidate for large-scale, low-cost photocatalytic water purification with commercial potential. Furthermore, our approach to transform WRN and IOS into a CQDs/FeOx composite opens new possibilities for the combined utilization of waste.
Abbreviations
| WRN | Waste rice noodle |
| IOS | Iron oxide scale |
| CQDs | Carbon quantum dots |
| Symbols | |
| CQDs/FeOx-x | Samples of CQDs/FeOx composites with different calcination temperatures (x = 1, 2, 3, 4, 5) and different carbon contents (x = 3, 6, 7) |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano13182506/s1. S1: General characterization; S2: Measurement of photocatalytic performance; Figure S1: The particle size distribution; Figure S2 Comparative images of photocatalytic degradation of methylene blue; Figures S3–S6: The kinetic fitting of photocatalytic degradation; Figure S7: The emission spectrum of the 405 nm purple light lamp; Tables S1 to S2: The kinetic parameters of photocatalytic degradation.
Author Contributions
Conceptualization, methodology and writing (original draft preparation): W.Y.; Software: Q.L.; Investigation: X.J.; Resources: G.D.; Formal Analysis: M.L.; Data curation: P.W.; Writing (review and editing), project administration, funding acquisition and supervision: S.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by grants from the National Nature Science Foundation of China (No. 51763007) and Guangxi Natural Science Foundation Program (No. 2015GXNSFBA139033).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zong X., Wang L. Ion-exchangeable semiconductor materials for visible light-induced photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2014;18:32–49. doi: 10.1016/j.jphotochemrev.2013.10.001. [DOI] [Google Scholar]
- 2.Xia G.M. Interdiffusion in group IV semiconductor material systems: Applications, research methods and discoveries. Sci. Bull. 2019;64:1436–1455. doi: 10.1016/j.scib.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Guo Y., Xu Z., Curto A.G., Zeng Y.-J., Van Thourhout D. Plasmonic semiconductors: Materials, tunability and applications. Prog. Mater. Sci. 2023;138:101158. doi: 10.1016/j.pmatsci.2023.101158. [DOI] [Google Scholar]
- 4.Dharani S., Vadivel S., Gnanasekaran L., Rajendran S. S-scheme heterojunction photocatalysts for hydrogen production: Current progress and future prospects. Fuel. 2023;349:128688. doi: 10.1016/j.fuel.2023.128688. [DOI] [Google Scholar]
- 5.Wang J., Wang Z., Dai K., Zhang J. Review on inorganic–organic S-scheme photocatalysts. J. Mater. Sci. Technol. 2023;165:187–218. doi: 10.1016/j.jmst.2023.03.067. [DOI] [Google Scholar]
- 6.Ruziwa D.T., Oluwalana A.E., Mupa M., Meili L., Selvasembian R., Nindi M.M., Sillanpaa M., Gwenzi W., Chaukura N. Pharmaceuticals in wastewater and their photocatalytic degradation using nano-enabled photocatalysts. J. Water Process Eng. 2023;54:103880. doi: 10.1016/j.jwpe.2023.103880. [DOI] [Google Scholar]
- 7.Yue C., Zhu L., Qiu Y., Du Z., Qiu J., Liu F., Wang F. Recent advances of plasmonic elemental Bi based photocatalysts in environmental remediation and energy conversion. J. Clean. Prod. 2023;392:136017. doi: 10.1016/j.jclepro.2023.136017. [DOI] [Google Scholar]
- 8.Noureen L., Wang Q., Humayun M., Shah W.A., Xu Q., Wang X. Recent advances in structural engineering of photocatalysts for environmental remediation. Environ. Res. 2023;219:115084. doi: 10.1016/j.envres.2022.115084. [DOI] [PubMed] [Google Scholar]
- 9.Shubha J.P., Roopashree B., Patil R.C., Khan M., Shaik M.R., Alaqarbeh M., Alwarthan A., Mahmoud Karami A., Adil S.F. Facile synthesis of ZnO/CuO/Eu heterostructure photocatalyst for the degradation of industrial effluent. Arab. J. Chem. 2023;16:104547. doi: 10.1016/j.arabjc.2023.104547. [DOI] [Google Scholar]
- 10.Wang M., Xu Z., Qi Z., Cai Y., Li G., Choi W., An T. Repeated photocatalytic inactivation of E. coli by UV + Ni foam@TiO2: Performance and photocatalyst deactivation. Chem. Eng. J. 2023;468:143680. doi: 10.1016/j.cej.2023.143680. [DOI] [Google Scholar]
- 11.Lu Y., Chen R., Wu F., Gan W., Zhao Z., Chen L., Zhang M., Sun Z. A novel SiP/TiO2 S-scheme heterojunction photocatalyst for efficient degradation of norfloxacin. Sep. Purif. Technol. 2023;324:124572. doi: 10.1016/j.seppur.2023.124572. [DOI] [Google Scholar]
- 12.Sadikin S.N., Ridwan J., Umar M.I.A., Raub A.A.M., Yunas J., Hamzah A.A., Dahlan D., Rahman M.Y.A., Umar A.A. Photocatalytic activity and stability properties of porous TiO2 film as photocatalyst for methylene blue and methylene orange degradation. Int. J. Electrochem. Sci. 2023;18:100246. doi: 10.1016/j.ijoes.2023.100246. [DOI] [Google Scholar]
- 13.Bi T., Du Z., Chen S., He H., Shen X., Fu Y. Preparation of flower-like ZnO photocatalyst with oxygen vacancy to enhance the photocatalytic degradation of methyl orange. Appl. Surf. Sci. 2023;614:156240. doi: 10.1016/j.apsusc.2022.156240. [DOI] [Google Scholar]
- 14.Sharma K., Raizada P., Hasija V., Singh P., Bajpai A., Nguyen V.-H., Rangabhashiyam S., Kumar P., Nadda A.K., Kim S.Y., et al. ZnS-based quantum dots as photocatalysts for water purification. J. Water Process Eng. 2021;43:102217. doi: 10.1016/j.jwpe.2021.102217. [DOI] [Google Scholar]
- 15.Ullah H., Haneef Z., Ahmad A., Butler I.S., Nasir Dara R., Rehman Z. MoS2 and CdS photocatalysts for water decontamination: A review. Inorg. Chem. Commun. 2023;153:110775. doi: 10.1016/j.inoche.2023.110775. [DOI] [Google Scholar]
- 16.López J., Rey A., Viñuelas-Zahinos E., Álvarez P.M. Preparation of a new green magnetic Fe3O4 @TiO2-P25 photocatalyst for solar advanced oxidation processes in water. J. Environ. Chem. Eng. 2023;11:109999. doi: 10.1016/j.jece.2023.109999. [DOI] [Google Scholar]
- 17.Waheed I.F., Hamad M.A., Jasim K.A., Gesquiere A.J. Degradation of methylene blue using a novel magnetic CuNiFe2O4/g-C3N4 nanocomposite as heterojunction photocatalyst. Diam. Relat. Mater. 2023;133:109716. doi: 10.1016/j.diamond.2023.109716. [DOI] [Google Scholar]
- 18.Jiang Z., Zou Y., Hao Y., Xu L., Liu C., Su H., Gong S. Magnetic recyclable ZnO/SrFe12O19 photocatalyst for effective photodegradation of rhodamine B under simulated sunlight. Int. J. Hydrog. Energy. 2022;47:34387–34396. doi: 10.1016/j.ijhydene.2022.08.038. [DOI] [Google Scholar]
- 19.Rafieezadeh M., Kianfar A.H. Fabrication of heterojunction ternary Fe3O4/TiO2/CoMoO4 as a magnetic photocatalyst for organic dyes degradation under sunlight irradiation. J. Photochem. Photobiol. A Chem. 2022;423:113596. doi: 10.1016/j.jphotochem.2021.113596. [DOI] [Google Scholar]
- 20.Dulyasucharit R., Wongkasemjit S., Nanan S., Intharaksa O., Masingboon C. Magnetic Fe3O4/Bi2O2(OH)(NO3) as a sunlight-driven photocatalyst for rhodamine B degradation. J. Solid State Chem. 2023;319:123784. doi: 10.1016/j.jssc.2022.123784. [DOI] [Google Scholar]
- 21.Li X., Qiu Y., Zhu Z., Chen T., Zhang H., Yin D. Construction of magnetically separable dual Z-scheme g-C3N4/α-Fe2O3/ Bi3TaO7 photocatalyst for effective degradation of ciprofloxacin under visible light. Chem. Eng. J. 2022;440:135840. doi: 10.1016/j.cej.2022.135840. [DOI] [Google Scholar]
- 22.Mohan H., Ramasamy M., Ramalingam V., Natesan K., Duraisamy M., Venkatachalam J., Shin T., Seralathan K.K. Enhanced visible light-driven photocatalysis of iron-oxide/titania composite: Norfloxacin degradation mechanism and toxicity study. J. Hazard. Mater. 2021;412:125330. doi: 10.1016/j.jhazmat.2021.125330. [DOI] [PubMed] [Google Scholar]
- 23.Subha N., Mahalakshmi M., Monika S., Senthil Kumar P., Preethi V., Vaishnavi G., Rajabhuvaneswari A. Heterostructured γ-Fe2O3/FeTiO3 magnetic nanocomposite: An efficient visible-light-driven photocatalyst for the degradation of organic dye. Chemosphere. 2022;306:135631. doi: 10.1016/j.chemosphere.2022.135631. [DOI] [PubMed] [Google Scholar]
- 24.Hayati F., Moradi S., Farshineh Saei S., Madani Z., Giannakis S., Isari A.A., Kakavandi B. A novel, Z-scheme ZnO@AC@FeO photocatalyst, suitable for the intensification of photo-mediated peroxymonosulfate activation: Performance, reactivity and bisphenol A degradation pathways. J. Environ. Manag. 2022;321:115851. doi: 10.1016/j.jenvman.2022.115851. [DOI] [PubMed] [Google Scholar]
- 25.Mishra S., Acharya R. Recent updates in modification strategies for escalated performance of Graphene/MFe2O4 heterostructured photocatalysts towards energy and environmental applications. J. Alloys Compd. 2023;960:170576. doi: 10.1016/j.jallcom.2023.170576. [DOI] [Google Scholar]
- 26.Sanei A., Dashtian K., Yousefi Seyf J., Seidi F., Kolvari E. Biomass derived reduced-graphene-oxide supported α-Fe2O3/ZnO S-scheme heterostructure: Robust photocatalytic wastewater remediation. J. Environ. Manag. 2023;332:117377. doi: 10.1016/j.jenvman.2023.117377. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z., Luo M., Yuan S., Meng L., Ding W., Su S., Cao Y., Wang Y., Li X. Boron-doped graphene quantum dot/bismuth molybdate composite photocatalysts for efficient photocatalytic nitrogen fixation reactions. J. Colloid Interface Sci. 2023;650:1301–1311. doi: 10.1016/j.jcis.2023.07.085. [DOI] [PubMed] [Google Scholar]
- 28.Mohamed M.M., Bayoumy W.A., Goher M.E., Abdo M.H., Mansour El-Ashkar T.Y. Optimization of α-Fe2O3@Fe3O4 incorporated N-TiO2 as super effective photocatalysts under visible light irradiation. Appl. Surf. Sci. 2017;412:668–682. doi: 10.1016/j.apsusc.2017.03.200. [DOI] [Google Scholar]
- 29.Sun A.-C. Synthesis of magnetic carbon nanodots for recyclable photocatalytic degradation of organic compounds in visible light. Adv. Powder Technol. 2018;29:719–725. doi: 10.1016/j.apt.2017.12.013. [DOI] [Google Scholar]
- 30.Li Y., Xiang W., Zhou T., Huang M., Wang C., Wu X., Mao J., Wang P. Visible light induced efficient activation of persulfate by a carbon quantum dots (CQDs) modified g-Fe2O3 catalyst. Chin. Chem. Lett. 2020;31:2757–2761. doi: 10.1016/j.cclet.2020.01.032. [DOI] [Google Scholar]
- 31.Huang S., Zhang Q., Liu P., Ma S., Xie B., Yang K., Zhao Y. Novel up-conversion carbon quantum dots/α-FeOOH nanohybrids eliminate tetracycline and its related drug resistance in visible-light responsive Fenton system. Appl. Catal. B Environ. 2020;263:118336. doi: 10.1016/j.apcatb.2019.118336. [DOI] [Google Scholar]
- 32.Mmelesi O.K., Ammar-Merah S., Nkambule T.T.I., Kefeni K.K., Kuvarega A.T. Synergistic role of N-doped carbon quantum dots on Zn-doped cobalt ferrite (N-CQDs/ZnCF) for the enhanced photodegradation of oxytetracycline under visible light. Mater. Sci. Eng. B. 2023;294:116538. doi: 10.1016/j.mseb.2023.116538. [DOI] [Google Scholar]
- 33.Wu X., Wang X., Lynch I., Guo Z., Zhang P., Wu L., Ning P., Ren N. Exceptional photo-elimination of antibiotic by a novel Z-scheme heterojunction catalyst composed of nanoscale zero valent iron embedded with carbon quantum dots (CQDs)-black TiO2. J. Hazard. Mater. 2023;304:132323. doi: 10.1016/j.jhazmat.2023.132323. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J., Liu R., Kuang M., Xie S., Wang J., Ji Z. Constructing CQDs/MIL-101(Fe)/g-C3N4 photocatalyst and its enhancement to the photocatalytic activity. Mater. Lett. 2023;167:135004. doi: 10.1016/j.matlet.2023.135004. [DOI] [Google Scholar]
- 35.Li Y., Jin Y., Li J., Chen Y., Gong Y., Li Y., Zhang J. Current situation and development of kitchen waste treatment in China. Procedia Environ. Sci. 2016;31:40–49. doi: 10.1016/j.proenv.2016.02.006. [DOI] [Google Scholar]
- 36.Deng Y., Chen X., Adam N.G.T.S., Xu J. A multi-objective optimization approach for clean treatment of food waste from an economic-environmental-social perspective: A case study from China. J. Clean. Prod. 2022;357:131559. doi: 10.1016/j.jclepro.2022.131559. [DOI] [Google Scholar]
- 37.Farahdiba A.U., Warmadewanthi I.D.A.A., Fransiscus Y., Rosyidah E., Hermana J., Yuniarto A. The present and proposed sustainable food waste treatment technology in Indonesia: A review. Environ. Technol. Innov. 2023;32:103256. doi: 10.1016/j.eti.2023.103256. [DOI] [Google Scholar]
- 38.Wang B., Ma J., Zhang L., Su Y., Xie Y., Ahmad Z., Xie B. The synergistic strategy and microbial ecology of the anaerobic co-digestion of food waste under the regulation of domestic garbage classification in China. Sci. Total Environ. 2021;765:144632. doi: 10.1016/j.scitotenv.2020.144632. [DOI] [PubMed] [Google Scholar]
- 39.Gao X., Yang F., Yan Z., Zhao J., Li S., Nghiem L., Li G., Luo W. Humification and maturation of kitchen waste during indoor composting by individual households. Sci. Total Environ. 2022;814:152509. doi: 10.1016/j.scitotenv.2021.152509. [DOI] [PubMed] [Google Scholar]
- 40.Li P., Ma J., Li L., Han Y., Zheng T., Wang Y., Chai F., Liu J. Emission behavior and impact assessment of gaseous volatile compounds in two typical rural domestic waste landfills. J. Environ. Manag. 2023;325:116659. doi: 10.1016/j.jenvman.2022.116659. [DOI] [PubMed] [Google Scholar]
- 41.Liu B., Han Z., Liang X. Dioxin emissions from municipal solid waste incineration in the context of waste classification policy. Atmos. Pollut. Res. 2023;14:101842. doi: 10.1016/j.apr.2023.101842. [DOI] [Google Scholar]
- 42.Mohanakrishna G., Sneha N.P., Rafi S.M., Sarkar O. Dark fermentative hydrogen production: Potential of food waste as future energy needs. Sci. Total Environ. 2023;888:163801. doi: 10.1016/j.scitotenv.2023.163801. [DOI] [PubMed] [Google Scholar]
- 43.Kumar V., Sharma N., Umesh M., Selvaraj M., Al-Shehri B.M., Chakraborty P., Duhan L., Sharma S., Pasrija R., Awasthi M.K., et al. Emerging challenges for the agro-industrial food waste utilization: A review on food waste biorefinery. Bioresour. Technol. 2022;362:127790. doi: 10.1016/j.biortech.2022.127790. [DOI] [PubMed] [Google Scholar]
- 44.Jin X., Che R., Yang J., Liu Y., Chen X., Jiang Y., Liang J., Chen S., Su H. Activated Carbon and Carbon Quantum Dots/Titanium Dioxide Composite Based on Waste Rice Noodles: Simultaneous Synthesis and Application in Water Pollution Control. Nanomaterials. 2022;12:472. doi: 10.3390/nano12030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin X.Y., Ying W.Y., Che R.J., Xiao P., Zhou Y.Q., Liu Y., Liu M.Y., Chen S.P. CQDs/ZnO composites based on waste rice noodles: Preparation and photocatalytic capability. RSC Adv. 2022;12:23692–23703. doi: 10.1039/D2RA03709B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jjagwe J., Olupot P.W., Carrara S. Iron oxide nanoparticles/nanocomposites derived from steel and iron wastes for water treatment: A review. J. Environ. Manag. 2023;343:118236. doi: 10.1016/j.jenvman.2023.118236. [DOI] [PubMed] [Google Scholar]
- 47.Yu J., Xu R., Zhang J., Zheng A. A review on reduction technology of air pollutant in current China’s iron and steel industry. J. Clean. Prod. 2023;414:137659. doi: 10.1016/j.jclepro.2023.137659. [DOI] [Google Scholar]
- 48.Hao J., Dou Z.-H., Zhang T.-A., Jiang B.-C., Wang K., Wan X.-Y. Manufacture of wear-resistant cast iron and copper-bearing antibacterial stainless steel from molten copper slag via vortex smelting reduction. J. Clean. Prod. 2022;375:134202. doi: 10.1016/j.jclepro.2022.134202. [DOI] [Google Scholar]
- 49.Rai S.K., Mukherjee A.K. Optimization for production of liquid nitrogen fertilizer from the degradation of chicken feather by iron-oxide (Fe3O4) magnetic nanoparticles coupled β-keratinase. Biocatal. Agric. Biotechnol. 2015;4:632–644. doi: 10.1016/j.bcab.2015.07.002. [DOI] [Google Scholar]
- 50.Cui F., Zhou S., Wang D., Tan X., Li Q., Li T., Li J. Preparation and antibacterial properties of amino functionalized antibacterial carbon quantum dots. Packag. Eng. 2023;44:121–129. [Google Scholar]
- 51.Wang L., Lu T., Ruan F., Deng D., Xu S. Synthesis of photoluminescent carbon nanoparticlesby hydrothermal method. Chin. J. Lumin. 2014;35:706–709. doi: 10.3788/fgxb20143506.0706. [DOI] [Google Scholar]
- 52.Graham M.J., Hussey R.J. The growth and structure of oxide films on Fe. I. oxidation of (001) and (112) Fe at 200–300 °C. Oxid. Met. 1981;15:407–420. doi: 10.1007/BF00603533. [DOI] [Google Scholar]
- 53.Chen R.Y., Yuen W.Y.D. Review of the high-temperature oxidation of iron and carbon steels in air or oxygen. Oxid. Met. 2003;59:433–468. doi: 10.1023/A:1023685905159. [DOI] [Google Scholar]
- 54.Atkinson A. Transport processes during the growth of oxide films at elevated temperature. Rev. Mod. Phys. 1985;57:437–470. doi: 10.1103/RevModPhys.57.437. [DOI] [Google Scholar]
- 55.Guo P., Shi W., Wang H., Han M., Li H., Hui H., Liu Y., Kang Z. Facile fabrication of a CoO/g-C3N4 p–n heterojunction with enhanced photocatalytic activity and stability for tetracycline degradation under visible light. Catal. Sci. Technol. 2017;7:3225–3331. doi: 10.1039/C7CY00960G. [DOI] [Google Scholar]
- 56.Swedha M., Okla M., Abdel-Maksoud M., Kokilavani S., Kamwilaisak K., Sudheer Khan S. Photo-Fenton system Fe3O4/NiCu2S4 QDs towards bromoxynil and cefixime degradation: A realistic approach. Surf. Interfaces. 2023;38:102764. doi: 10.1016/j.surfin.2023.102764. [DOI] [Google Scholar]
- 57.Harikumar B., Okla M., Kokilavani S., Almunqedhi B., Alshuwaish R., Abdel-Maksoud M., El-Tayeb M., Sudheer Khan S. Insights into oxygen defect enriched and non-metal dopant co modulated Fe3O4 nanospheres embedded WO3 nanorods for ameliorated photodegradation of doxycycline, Cr(VI) reduction and its genotoxicity. J. Clean. Prod. 2023;398:136549. doi: 10.1016/j.jclepro.2023.136549. [DOI] [Google Scholar]
- 58.Alexpandi R., Abirami G., Murugesan B., Durgadevi R., Swasthikka R.P., Cai Y., Ragupathi T., Ravi A.V. Tocopherol-assisted magnetic Ag-Fe3O4-TiO2 nanocomposite for photocatalytic bacterial-inactivation with elucidation of mechanism and its hazardous level assessment with zebrafish model. J. Hazard. Mater. 2023;442:130044. doi: 10.1016/j.jhazmat.2022.130044. [DOI] [PubMed] [Google Scholar]
- 59.Jubu P.R., Yam F.K., Igba V.M., Beh K.P. Tauc-plot scale and extrapolation effect on bandgap estimation from UV–vis–NIR data–A case study of β-Ga2O3. J. Solid State Chem. 2020;290:121576. doi: 10.1016/j.jssc.2020.121576. [DOI] [Google Scholar]
- 60.Jubu P.R., Obaseki O.S., Nathan-Abutu A., Yam F.K., Yusof Y., Ochang M.B. Dispensability of the conventional Tauc’s plot for accurate bandgap determination from UV–vis optical diffuse reflectance data. Results Opt. 2022;9:100273. doi: 10.1016/j.rio.2022.100273. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.