Figure 2.
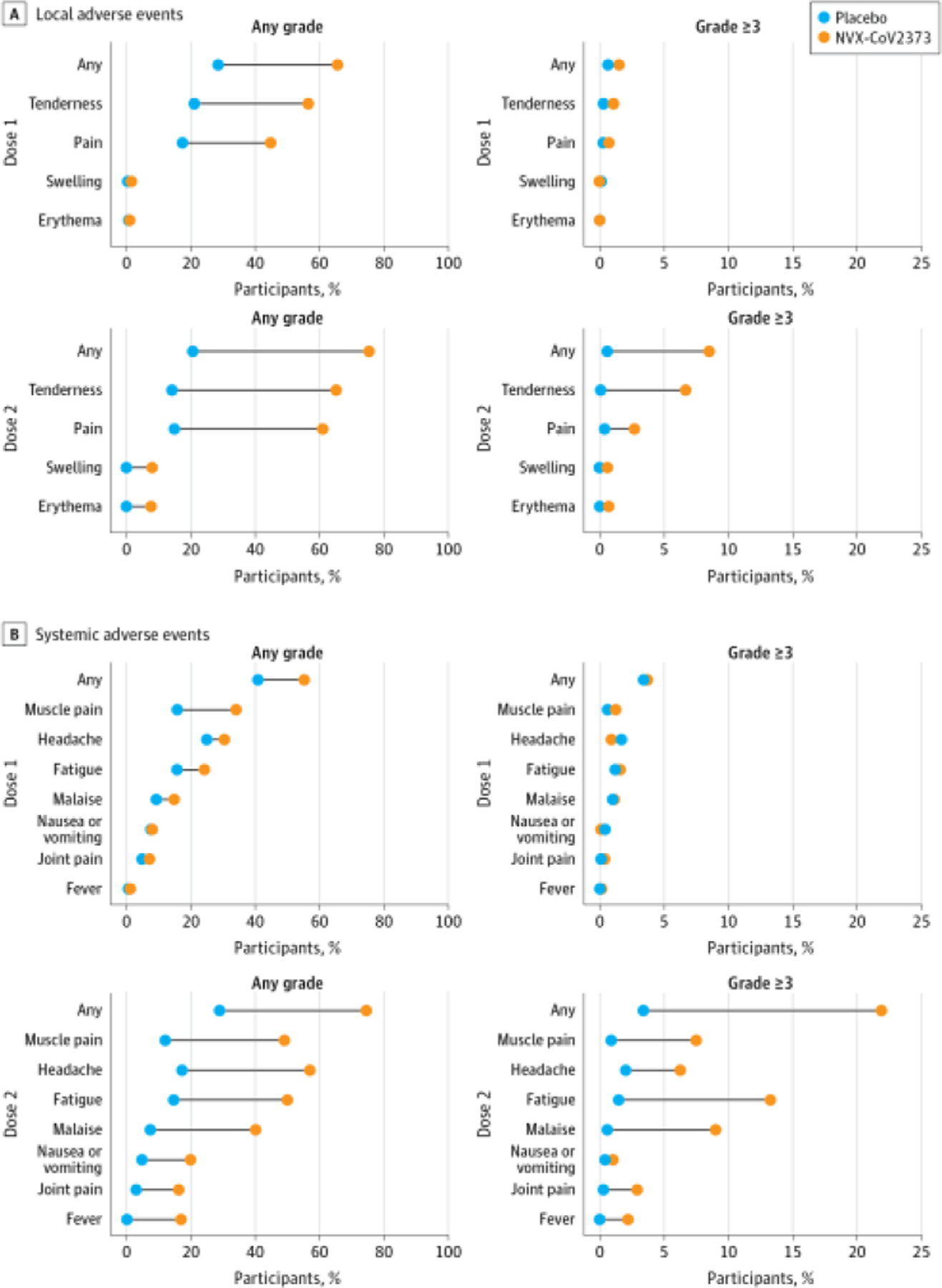
Solicited Local and Systemic Adverse Events
The percentage of participants in each treatment group with solicited local (A) and systemic (B) adverse events during the 7 days after each vaccination is plotted by US Food and Drug Administration toxicity grade, as any (mild, moderate, severe, or potentially life-threatening) or as grade 3 or higher (severe or potentially life-threatening).21
