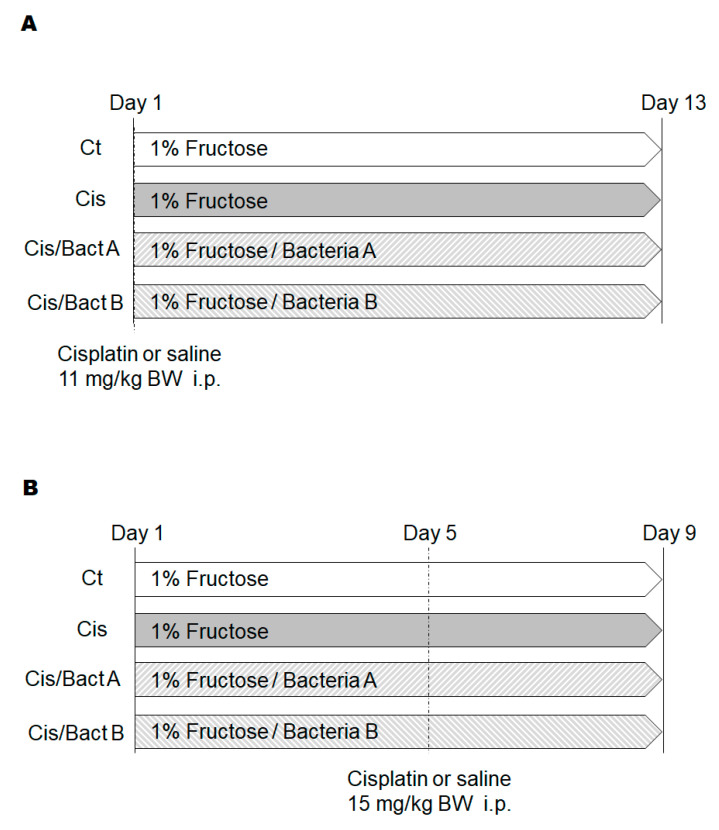
Figure 2.
Study design. (A) Male ICR mice (6 weeks old) were separated into four groups: Ct (control, n = 4), Cis (cisplatin-induced kidney injury, n = 6), Cis/Bact A (cisplatin-induced kidney injury/bacteria A, n = 6), Cis/Bact B (cisplatin-induced kidney injury/bacteria B, n = 6). On day 5, the Ct group received 15 mg/kg B.W. of saline intraperitoneally, and the Cis, Cis/Bact A and Cis/Bact B groups received 15 mg/kg B.W. of cisplatin intraperitoneally. All mice were sacrificed on day 9. (B) Male ICR mice (6 weeks old) were divided into four groups: Ct (control, n = 4), Cis (cisplatin-induced kidney injury, n = 6), Cis/Bact A (cisplatin-induced kidney injury/bacteria A, n = 6), Cis/Bact B (cisplatin-induced kidney injury/bacteria B, n = 6). On day 5, the Ct group received 15 mg/kg B.W. of saline intraperitoneally, and the Cis, Cis/Bact A and Cis/Bact B groups received 15 mg/kg B.W. of cisplatin intraperitoneally. All mice were sacrificed on day 9.