Figure 2. Mechanisms of FGFR1 nuclear translocation and nuclear FGFR1 activity in breast cancer.
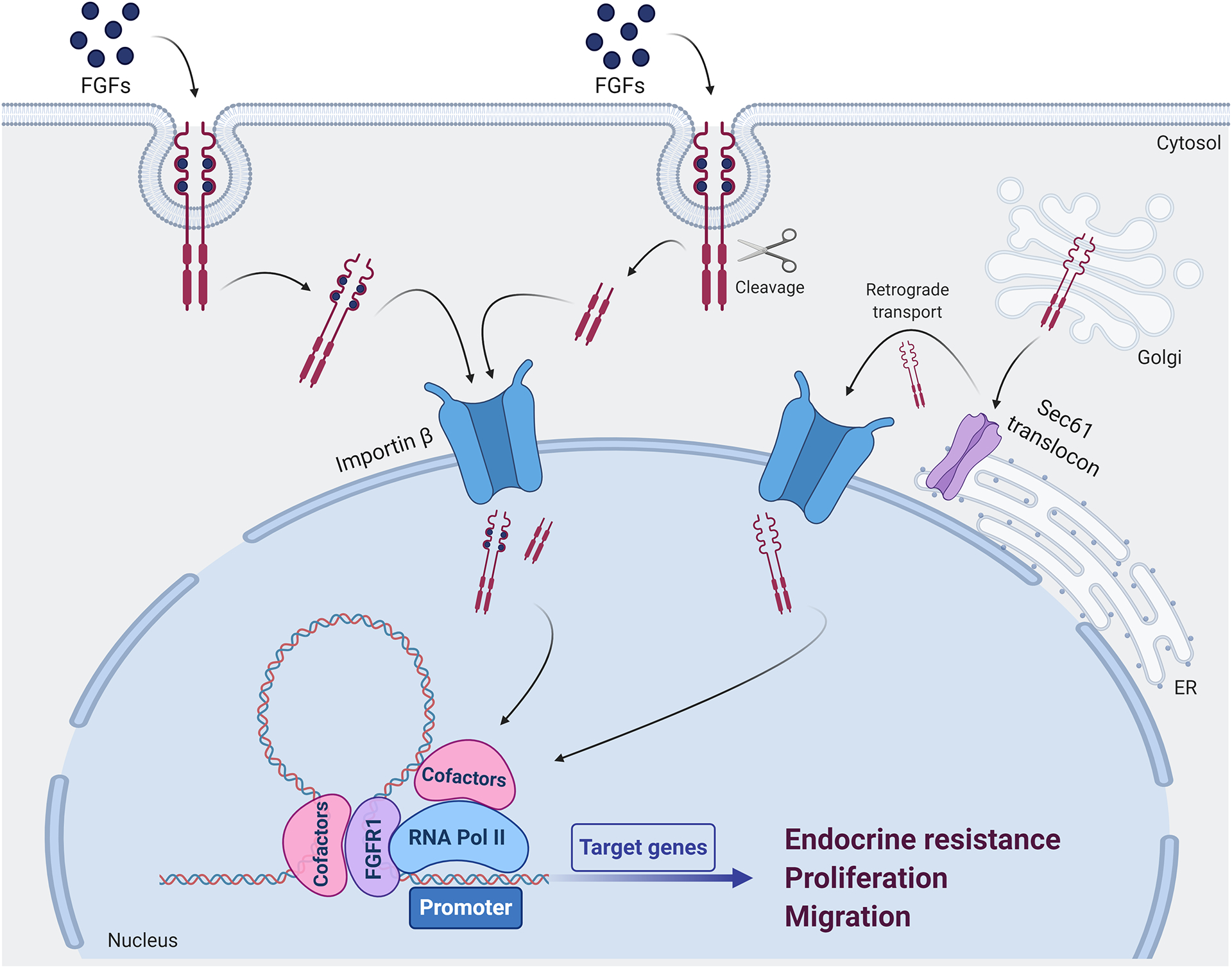
Following FGFs binding, full-length FGFR1 can undergo clathrin-mediated or clathrin independent endocytosis. In addition, upon FGFs binding, the intracellular domain of FGFR1 can be subjected to the proteolytic activity of granzyme B, which induces cleavage of the receptor recognizing the Asp432 residue. Both full-length and cleaved FGFR1 migrate into the nucleus via Importin β system. Furthermore, newly synthesized FGFR1 can be directed into the nucleus via a retrograde transport system involving the ER-associated Sec61 translocon and Importin β. In the nucleus, FGFR1 associates with RNA Polymerase II in a chromatin-bound complex of coregulators and contributes to regulate the transcription of genes associated with endocrine resistance, cell proliferation and migration. FGFs: fibroblast growth factors; ER: endoplasmic reticulum; RNA Pol II: RNA polymerase II.
