Figure 3 |. Targeting lymph node-resident cells and lymph node subregions.
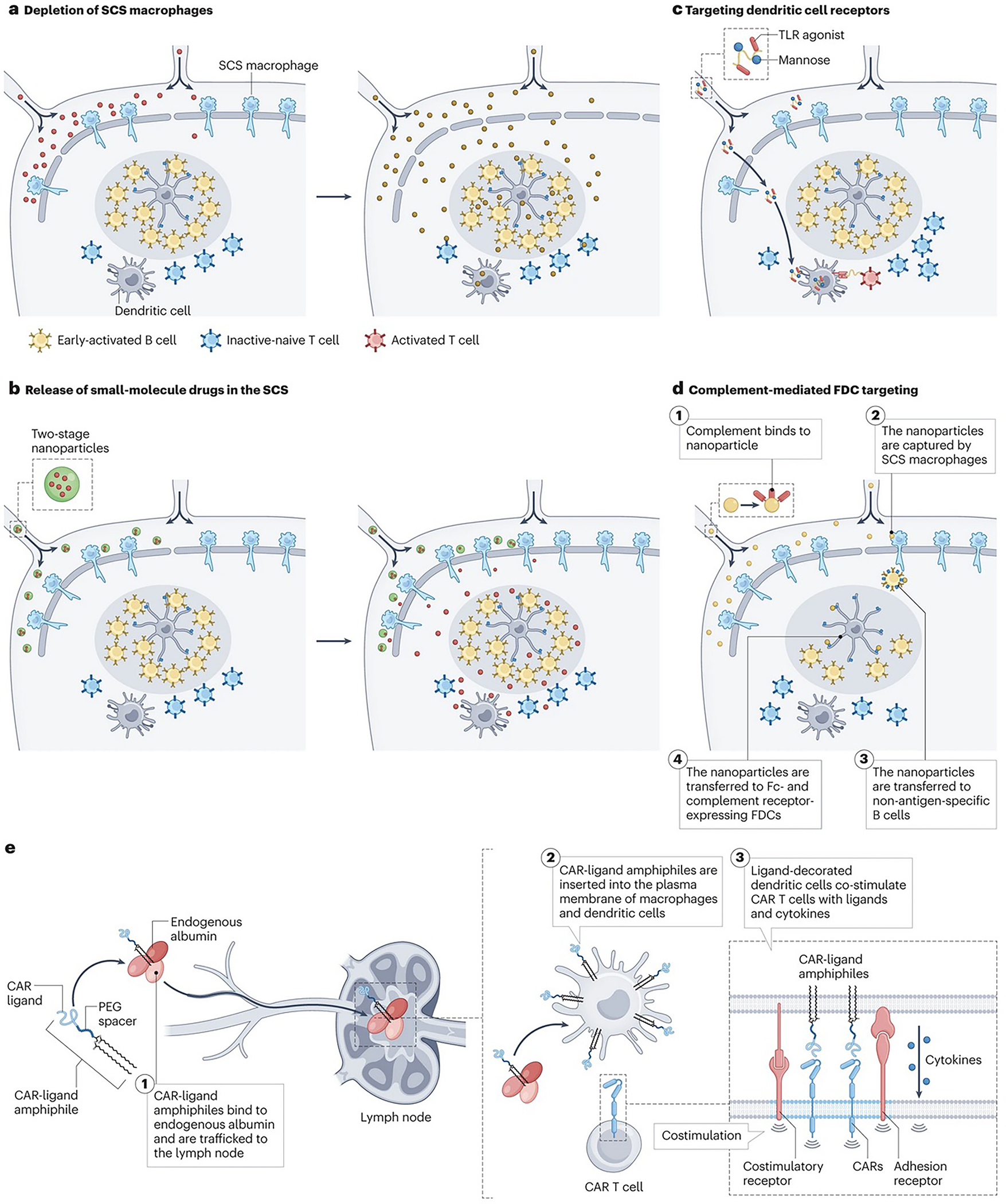
a | Promoting therapeutic entry into lymph nodes (LNs) through depletion of subcapsular sinus (SCS) macrophages. Vaccine adjuvants (red) induce rapid death of SCS macrophages, leading to enhanced entry of antigens and other compounds (orange) into LNs. b | Polymer nanoparticles (blue) efficiently traffic into lymphatic vessels (20–30 nm) but are too large to penetrate the LN paracortex. They chemically release small molecule payloads (red) that rapidly permeate throughout the node. c | Soluble polymers and polymer nanoparticles (NPs) conjugated with ligands for receptors expressed by specific target cell types (such as dendritic cells (DCs)), are taken up by antigen presenting cells (APCs) in LNs and co-deliver vaccine antigens or DC activating compounds. d | (1) NPs activate complement, (2) are captured by SCS macrophages (3) and transferred to non-antigen-specific B cells through complement receptors. (4) These NPs are then transferred to follicular dendritic cells (FDCs), which express high levels of complement and (fragment crystallisable) Fc receptors. e | (1) Ligands for chimeric antigen receptor (CAR) T cells linked to albumin-binding poly(ethylene glycol) (PEG)-lipid moieties traffic from an injection site and bind to endogenous albumin. These compounds are then transferred to LNs (2) where they are inserted into the plasma membrane of macrophages and DCs. Subsequent encounter of CAR T cells with the ligand displayed on the surface of DCs will lead to CAR T cell tandem stimulation by natural costimulatory receptors and cytokine signals provided by the ligand-decorated DCs.
