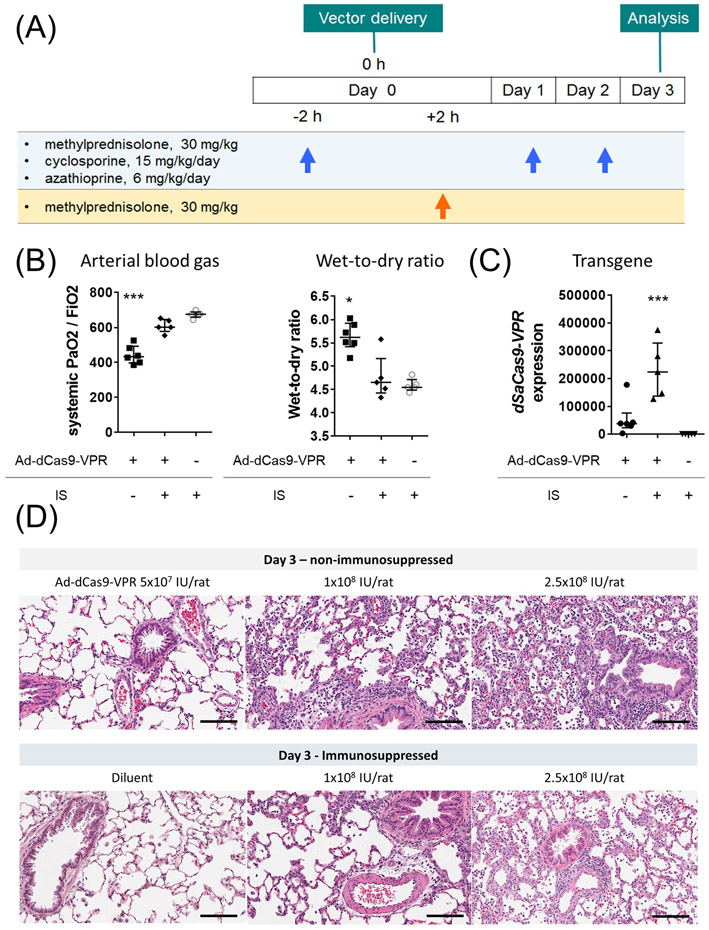
Figure 3.
Impact of triple immunosuppression on ameliorating inflammation after dSaCas9-VPR delivery to the lung. (A) Experimental design and immunosuppression protocol. Immunosuppressants were intraperitoneally injected. (B, C) The left lungs were treated with Ad-dSaCas9-VPR at a dose of 2.5x108 IU/rat and analyzed on day 3. Systemic PaO2 was measured using arterial blood taken from abdominal aorta. Wet-to-dry ratio and dSaCas9 expression were measured using lung tissues collected (Ad-dCas9-VPR without IS, n=6; others, n=5). Median (interquartile range) are presented. A Kruskal-Wallis test followed by Dunn’s correction was used for comparison. *: p ≤ 0.05, ***: p ≤ 0.001. (D) Representative images of hematoxylin and eosin staining of the lung tissues treated with different doses of Ad-dCas9-VPR with or without immunosuppression. Scale bars, 100 μm. IS: immunosuppression.