Abstract
Per- and polyfluoroalkyl substances (PFAS) exposure has been associated with reduced antibody levels. Higher red blood cell (RBC) folate was previously associated with lower serum PFAS concentrations in adolescents. This study included 819 adolescents aged 12 to 19 years who had detectable rubella and measles antibody levels in serum from the U.S. National Health and Nutrition Examination Survey 2003–2004 and 2009–2010 cycles. We found inverse associations between serum PFOS and PFHxS and rubella antibodies, between PFOA and mumps antibodies, and between PFAS mixtures and rubella and mumps antibodies, only among adolescents with RBC folate concentrations < 66th percentile (lower folate group) while not among adolescents with higher RBC folate levels (upper folate group). Specifically, per quartile increase in serum concentrations of the total PFAS mixture was associated with a 9.84% (95% CI: −15.57%, −3.74%) decrease in rubella antibody and an 8.79% (95% CI: −14.39%, −2.82%) decrease in the mumps antibody concentrations only in the lower folate group, while null associations were found for the upper folate group. If confirmed in mechanistic studies or prospective epidemiologic studies, these findings may have important implications for using folate as a mitigation measure against immune-related PFAS effects.
Keywords: folate, PFAS, immune, antibody, mitigation, children
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a class of manmade fluorinated chemicals, which are extremely persistent in the environment.1 Because of their water- and oil- resistance properties, PFAS has been widely applied in numerous commercial and industrial applications such as non-stick pans and kitchen supplies, water/oil resistant fabric, firefighting foams, and food packaging.2 Studies have linked PFAS exposure to a wide range of deleterious health outcomes in humans, including metabolic syndrome, adverse birth outcomes, altered immune function, and cancer.3–5
Consistent evidence supports that prenatal and childhood PFAS exposure is associated with decreased diphtheria antibody levels in children.6–8 One study used data from the National Health and Nutrition Examination Survey (NHANES) and reported that serum PFOS concentrations were negatively associated with rubella antibody concentrations among adolescents aged 12–19 years.9 No study examined the joint effect of the total PFAS mixture on antibody concentrations despite the fact that humans are exposed to multiple PFAS compounds simultaneously.10 The potential for PFAS mixtures to exert additive, synergistic, or antagonist effects needs further investigation.10
Our previous work found that higher folate levels measured in either diet, serum, or red blood cells (RBC) were consistently associated with lower serum PFAS concentrations after adjusting for important confounders including diet.11,12 Folate plays a critical role in maintaining normal function of the immune system,13 and increasing evidence supports its ability to mitigate harmful health effects of several environmental pollutants.14–16 Therefore, further investigation on whether folate status can modify the relationships between PFAS exposure and immune outcomes in children is warranted. Examining folate as an effect modifier not only helps identify vulnerable populations to PFAS-related adverse immune outcomes but also informs possible real-world interventions to mitigate harmful PFAS-related effects given the relatively easy access to folic acid supplementation. As such, this study aimed to examine the associations of serum concentrations of individual PFAS compounds as well as the total PFAS mixture in relation to rubella, measles, and mumps antibody levels, and to further evaluate if RBC folate modifies associations among adolescents aged 12–19 years in the NHANES 2003–2004 and 2009–2010 cycles.
Methods
Population
The NHANES is a cross-sectional U.S. representative study administered every two years with the aim to assess the health and nutritional conditions of the U.S. general population. Data are collected on anthropometrics, demographic, diet, and lifestyle factors via self-reported questionnaires, and participants provide urine and peripheral blood samples. Detailed information on NHANES study design can be found online.17
The present analysis included 819 adolescents aged 12 to 19 years who had complete measurements of serum PFAS concentrations, RBC folate concentrations, and serum antibody concentrations to rubella, measles, and mumps antibodies from the NHANES 2003–2004 and 2009–2010 cycles. We restricted analyses to adolescents who had detectable antibody levels to both measles and rubella in blood as a proxy for having measles-mumps-rubella (MMR) vaccination, to reduce confounding by vaccination and health consciousness, because participants who had vaccination might be more health conscious and thus have different levels of PFAS exposure and immune outcomes than people who did not have vaccination. We did not include the detection of mumps antibody as a criterion since the seroconversion rate of mumps from MMR vaccination is lower compared with that for measles and rubella.18 The participant inclusion flowchart is shown in Figure S1.
PFAS and Folate Measurements
Peripheral blood samples were collected at Mobile Examination Centers (MEC) for all NHANES study participants.19 Serum PFAS concentrations were measured in a random sample of around 1/3 of the total adolescent population in each cycle. On-line solid-phase extraction coupled to high-performance liquid chromatography–isotope dilution–tandem mass spectrometry (online SPE-HPLC–MS/MS) was employed to measure the serum concentrations of four PFAS compounds, including perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), perfluorohexane sulfonic acid (PFHxS), and perfluorononanoic acid (PFNA). Limits of detection (LOD) for the four compounds changed slightly between the two cycles (Table S1). Concentrations below the LOD were imputed by the LOD value divided by the squared root of 2 (imputed for < 2% of study participants).20,21
In the 2003–2004 cycle, RBC folate concentrations were measured using the Bio-Rad Laboratories “Quantaphase II Folate” radioassay kit. In the 2009–2010 cycle, RBC folate concentrations were calculated from serum folate and whole-blood folate concentrations measured in microbiologic assay as [(whole blood lysate folate * 11- serum folate (1 - hematocrit/100))/ (hematocrit /100)].22 Detailed laboratory and quality control procedures for PFAS and folate measurements can be found in NHANES laboratory protocols.22–25
Antibody Measurement
In the 2003–2004 cycle, serum IgG antibody levels to rubella, measles, and mumps viruses were measured with enzyme immuno-assay tests developed by the Immunoserology Unit of the California State Department of Health Services (CSDHS), Viral and Rickettsial Disease Laboratory (VRDL).26 In the 2009–2010 cycle, the Wampole IgG enzyme-linked immunosorbent assay II test system was used to detect the serum antibodies to rubella, measles, and mumps. In both cycles, the optical density index (OD index) was reported for the antibody levels of each virus. The international unit (IU) of rubella antibody level was converted from OD index by [(65.93928 × Rubella OD Index) * (0.177) + 1.28060] for the 2003–2004 cycle and [Rubella OD Index * 9.091] IU/mL for the 2009–2010 cycle. For both cycles, measles and mumps antibody OD index ≥ 1.1 or rubella antibody IU/mL ≥ 10 was considered as detectable of measles, mumps, and rubella antibody in serum respectively. Detailed laboratory and quality control information for antibody assessments can be found in the NHANES laboratory procedures.27–30
Covariates
Data on demographic characteristics, including age (continuous), sex (dichotomous), race (Non-Hispanic White, Non-Hispanic Black, Hispanic, Other Race), and household income were collected by self-reported questionnaires. Income-poverty ratio was the ratio of self-reported household income and the income for the poverty line. A ratio < 1 indicated that the participant lived below the poverty threshold. Participant’s weight and height were measured by NHANES study staff. Body mass index (BMI) in kg/m2 was calculated as weight (kg) divided by the squared height (m2). Dietary information was obtained by averaging data from two dietary interviews, which spanned 3–10 days in time, on recalls of the diet in the past 24 hours prior to the interviews. Additional information on seafood consumption in the past 30 days was added to the dietary interview in the 2009–2010 cycle. Serum cotinine concentration (ng/ml) was measured by an isotope-dilution high-performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometric (ID HPLC-APCI MS/MS) method. Participants with serum cotinine concentrations > 10 ng/ml were considered as exposed to tobacco smoke.31
Statistical Analyses
We stratified the study population into lower (bottom two tertiles) vs. upper folate group with the highest tertile of the survey cycle-specific RBC folate levels as the cut-point (234 ng/ml for the 2003–2004 cycle, 441.5 ng/ml for the 2009–2010 cycle). We conducted descriptive analyses for participants’ characteristics and distributions of serum PFAS and rubella, measles, and mumps antibody concentrations for the total population and the lower and upper folate groups, respectively. We also reported cycle-specific descriptive statistics for population characteristics and PFAS and antibody distributions.
In the associational analyses, PFAS and antibody levels were natural log-transformed to normalize the distributions, reduce the influence of outliers, and improve the interpretations of the associational results. In brief, we examined the associations for serum concentrations of individual PFAS compounds (single chemical analyses) as well as PFAS mixtures (mixture analyses) in relation to the three antibody concentrations in the total population and by the lower and upper folate groups, respectively.
Single Chemical Analyses
We used multivariable linear regressions to examine the associations between serum concentrations of individual PFAS compounds and antibody levels of rubella, measles, or mumps in the total population, and lower and upper folate groups, respectively. We exponentiated the beta coefficients to obtain the adjusted percent changes (PC) in rubella, measles, and mumps antibody levels per 2.7-fold increase in the serum concentrations of individual PFAS compound.
To examine the effect measure modification (EMM) by folate groups on the association between individual PFAS compounds and antibody level, we added an interaction term of folate group (lower vs. upper) * individual PFAS compound concentration in the regression model, the p value of which was reported as the EMM p value. An EMM p value ≤0.05 was considered statistically significant for EMM on the multiplicative scale. Adjusted covariates were selected a priori using a Directed Acyclic Graph (DAG) that included age (continuous), sex (binary), race (categorical), income-poverty ratio (continuous), BMI (continuous), serum cotinine concentrations (continuous), survey cycle (categorical), and dietary intake of milk and milk products, eggs, and meat (continuous), which were previously reported to be important sources of PFAS exposure 32–34 and can influence the immune function.35,36
Mixture Analyses
We utilized two mixture methods - quantile g-computation (QGC) 37 and Bayesian Kernel Machine Regression (BKMR) 38 – to evaluate the joint effect of the PFAS mixture on measles, rubella, or mumps antibody levels for the total population and the lower and upper folate groups, respectively.
In brief, QGC models examined the joint effect of the PFAS mixture on the natural log-transformed antibody levels. We exponentiated the beta coefficients to report the percent changes in antibody levels per quartile increase in the PFAS mixture with the first quartile taken as the reference group, adjusting for covariates. QGC also estimated the weight of individual PFAS compounds on the total joint effect (accommodating associations going in either positive or negative directions), which reflected the relative contribution of the individual PFAS compound to the total mixture effect. QGC models also evaluated the EMM and its p value by folate groups on the joint effect of the total PFAS mixture on antibody levels.
We complemented QGC analyses with BKMR models, which are based on a flexible kernel model that allows us to investigate non-linear relationships and complex interactions within the mixture. BKMR models reported 1) dose-response relationships between individual PFAS compounds and the natural log-transformed antibody levels, holding the rest of the PFAS compounds in the mixture at their median levels, and 2) joint effect of the PFAS mixture on the outcome by reporting the changes in natural log-transformed antibody concentration per 5th percentile increase or decrease from the median concentrations of the PFAS mixture, both conditioning on covariates. BKMR models also assessed the relative importance of the individual PFAS compound regarding the joint effect by using Posterior Inclusion Probabilities (PIPs). However, BKMR is unable to assess the EMM p value by folate status in contrast to QGC.
Sensitivity Analyses
To test the robustness of the cut-off for the RBC folate level in defining folate groups, we redefined the lower vs. upper folate group by the median (below vs. above median) and the lowest tertile (lowest vs. upper two tertiles) of cycle-specific RBC folate concentrations as cut-offs and repeated the primary stratified analyses of single-chemical analyses and QGC for the newly defined folate groups respectively.
Given the heterogeneity in antibody quantification methods in the two cycles, we stratified the analyses by the two cycles (2003–2004 vs. 2009–2010) to report cycle-specific associations between PFAS exposure and the three antibody levels. We further adjusted the 2009–2010 cycle analyses for seafood consumption in the past 30 days (Yes vs. No), which was only available for participants in the 2009–2010 cycle, for it is a major source of PFAS exposure and is beneficial to immune function.39,40
We further employed generalized additive models (GAM) to test the non-linear variations of the PFAS - antibody associations across RBC folate levels. We assumed a linear relationship between PFAS and antibody levels and allowed this linear relationship to vary smoothly across RBC folate concentrations. We fit the GAM models only to the 2003–2004 cycle data since this was the cycle that drove the overall associations (post-hoc knowledge), and only for significant PFAS-antibody associations.
We accounted for the complex NHANES design of sampling, clustering, and weighting in the analyses using the “survey” package (version 4.1.1) in R to obtain U.S. representative estimates, except for the mixture models and GAM due to technical limitations. We used R packages “qgcomp” (version 2.8.5) and “qgcompint” (version 0.6.6) for QGC models, “bkmr” (version 0.2.0) packages for BKMK models, and “gam” (version 1.20) for GAM. All analyses were conducted in R version 4.0.3 (R Development Core Team 2020).
Results
Population
The mean (SD) age of the study population was 15.50 (2.28) years with 50.22% of the adolescents being male. Most of the participants were non-Hispanic white (60.25%) (Table 1). Comparing the lower (< highest tertile) and the upper folate (≥ highest tertile) groups, the lower folate group was more racially diverse (non-Hispanic white: 52.74% in the lower folate group vs. 73.03% in the upper folate group), had more people living below the poverty line (25.10% vs. 17.05%) and exposed to tobacco smoke (15.27% vs. 7.04%) (Table 1). Compared with participants in the 2009–2010 cycle, participants in the 2003–2004 cycle were more likely to live under poverty line (24.81% vs. 19.47%) and exposed to tobacco smoke (15.06% vs. 9.40%) (Table S2).
Table 1.
Characteristics of adolescents aged 12 to 19 years in the United States, stratified by folate groups.
| Characteristics | Total Population N=819 | Lower Folate Group a N=552 | Upper Folate Group a N=267 |
|---|---|---|---|
|
| |||
| Age (years), mean (SD) | 15.50 (2.28) | 15.63 (2.24) | 15.24 (2.34) |
| BMI (kg/m2), mean (SD) | 23.89 (5.70) | 23.62 (5.37) | 24.44 (6.33) |
| Male, n (%) | 445 (50.22) | 299 (49.82) | 146 (50.87) |
| Race, n (%) | |||
| Non-Hispanic White | 235 (60.25) | 130 (52.74) | 105 (73.03) |
| Non-Hispanic Black | 253 (14.60) | 202 (18.94) | 51 (7.38) |
| Hispanic | 288 (17.88) | 187 (19.60) | 101 (15.02) |
| Other | 43 (7.17) | 33 (8.73) | 10 (4.57) |
| Income-Poverty Ratio, n (%) b | |||
| <1 | 269 (22.09) | 187 (25.10) | 82 (17.05) |
| 1=<, <2 | 240 (22.96) | 166 (24.38) | 74 (20.60) |
| 2=< | 310 (54.95) | 199 (50.51) | 111 (62.34) |
| Serum cotinine concentration (ng/ml), median (interquartile range) | 0.096 (0.021, 0.999) | 0.124 (0.024, 1.32) | 0.055 (0.011–0.664) |
| Exposed to tobacco smoke, n (%) c | 95 (12.18) | 76 (15.27) | 19 (7.04) |
Note. BMI, Body Mass Index.
The cut-off for defining lower vs. upper folate groups was the highest tertile of the cycle-specific red blood cell folate concentration. Cutoff = 234 ng/ml for 2003–2004 cycle, 441.5 ng/ml for 2009–2010 cycle.
Income-poverty ratio was the ratio of self-reported household income and the income of poverty guidelines.
Participants with serum cotinine concentration > 10 ng/ml were considered as exposed to tobacco smoke.
PFAS and Antibody Distributions
The four examined PFAS compounds were detected in almost all participants (detection rates > 98% in the total population). The detection rates, means, and interquartile ranges for the serum PFAS concentrations were similar in the lower and upper folate groups, though the upper folate group showed slightly lower PFAS concentrations (Table S3). The distributions of measles, rubella, and mumps antibody concentrations were similar in the lower and upper folate groups (Table S3). The seropositive rate of mumps antibody was 93.46% in the total study population, and was slightly higher in the lower folate group than the upper folate group (94.34% vs. 91.63%) (Table S3). Compared with the 2009–2010 cycle, serum concentrations of PFOA, PFOS, and PFHxS, measles and rubella antibody levels, and the seropositive rate of mumps antibody were higher in the 2003–2004 cycle (Table S4).
Single Chemical Associations
Rubella
We found an imprecise negative association between serum PFOA concentrations and rubella antibody levels in the total population (PC: −4.36%, 95% CI: −11.53%, 3.40%), which was substantially strengthened in the lower folate group (PC: −10.87%, 95% CI: −19.27%, −1.61%), while no meaningful association was found in the upper folate group (PC: 3.30%, 95% CI: −5.38%, 12.78%). The EMM by folate groups for the association between PFOA and rubella antibody levels was statistically significant (EMM p value =0.03) (Table 2).
Table 2.
Percent change in antibody levels per 2.7-fold increase in serum per- and polyfluoroalkyl substances among adolescents aged 12–19 years in the United States, stratified by folate groups.
| Total Population N=819 a | Lower Folate Group N= 552 a | Upper Folate Group N= 267 a | ||
|---|---|---|---|---|
| Percent Change (95% CI) b | Percent Change (95% CI) b | Percent Change (95% CI) b | EMM p value c | |
|
| ||||
| Rubella antibody | ||||
| PFOA | −4.36 (−11.53, 3.4) | −10.87 (−19.27, −1.61) | 3.3 (−5.38, 12.78) | 0.03 |
| PFOS | −8.16 (−13.67, −2.31) | −11 (−18.08, −3.31) | −5.51 (−12.09, 1.56) | 0.22 |
| PFHxS | −6.48 (−10.69, −2.07) | −7.22 (−11.62, −2.6) | −4.91 (−11.9, 2.64) | 0.53 |
| PFNA | 0.96 (−6.31, 8.8) | −1.37 (−10.52, 8.71) | 6.55 (−6.36, 21.24) | 0.39 |
| PFAS mixture d | −7.98 (−13.01, −2.66) | −9.84 (−15.57, −3.74) | −4.79 (−13.31, 4.57) | 0.32 |
| Mumps antibody | ||||
| PFOA | −11.05 (−18.56, −2.85) | −14.79 (−24.46, −3.89) | 24.36 (−0.09, 54.8) | 0.01 |
| PFOS | −2.12 (−8.11, 4.25) | −3.58 (−12.5, 6.24) | 11.48 (−3.88, 29.28) | 0.05 |
| PFHxS | −1.09 (−5.13, 3.12) | −4.77 (−10.16, 0.95) | 4.53 (−5.92, 16.14) | 0.13 |
| PFNA | −3.71 (−10.83, 3.97) | −3.82 (−10.17, 2.97) | 18.64 (−1.85, 43.41) | 0.07 |
| PFAS mixture d | −5.44 (−10.44, −0.16) | −8.79 (−14.39, −2.82) | 1.62 (−7.18, 11.25) | 0.04 |
| Measles antibody | ||||
| PFOA | −1.94 (−14.64, 12.64) | −11.44 (−25.94, 5.91) | 9.63 (−7.95, 30.56) | 0.09 |
| PFOS | −2.38 (−11.94, 8.21) | −2.52 (−15.37, 12.29) | −1.69 (−13.3, 11.48) | 0.91 |
| PFHxS | 0 (−6.8, 7.3) | 1.5 (−6.76, 10.49) | −2.52 (−12.37, 8.44) | 0.53 |
| PFNA | −1.76 (−11.88, 9.51) | −7.72 (−19.63, 5.96) | 12.33 (−5.51, 33.54) | 0.11 |
| PFAS mixture d | 7.03 (0.29, 14.22) | 5.64 (−2.08, 13.98) | 9.54 (−1.73, 22.11) | 0.57 |
Note. PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonic acid; PFHxS, perfluorohexane sulfonic acid; PFNA, perfluorononanoic acid; PFAS, per- and polyfluoroalkyl substances; EMM, effect measure modification.
The cut-off for defining lower vs. upper folate groups was the highest tertile of the cycle-specific red blood cell folate concentration. Cutoff = 234 ng/ml for 2003–2004 cycle, 441.5 ng/ml for 2009–2010 cycle. There were 8 observations with missing values for mumps antibody levels. For mumps analyses, N = 548 for the lower folate group, N = 263 for the upper folate group.
Models were adjusted for age (continuous), sex (dichotomous), race (categorical), income-poverty ratio (continuous), BMI (continuous), serum cotinine concentrations (continuous), survey cycle (categorical), and dietary intake of milk and milk products, eggs, and meat (continuous).
EMM p value is the p value for the interaction term of individual PFAS compound * folate group (low vs. upper).
Results from quantile g-computation.
We also observed significant negative associations between serum PFOS and PFHxS concentrations and rubella antibody levels in the total population (Table 2). After stratifying the analyses by lower and upper folate groups, the negative associations for rubella antibody levels remained only among those in the lower folate group (PC for PFOS: −11.00%, 95% CI:−18.08%, −3.31%; PC for PFHxS: −7.22%, 95% CI: −11.62%, −2.6%), while results became imprecise and weaker in the upper folate group (PC for PFOS: −5.51%, 95% CI: −12.09%, 1.56%; PC for PFHxS: −4.91%, 95% CI:−11.9%, 2.64%) (EMM p value: 0.22 for PFOS, 0.53 for PFHxS).
Mumps
We observed significant negative associations between serum PFOA concentration and mumps antibody levels in the total population (PC: −11.05%, 95% CI: −18.56%, −2.85%) (Table 2). Similar to findings on rubella antibodies, the negative association between serum PFOA and mumps antibodies was only found in the lower folate group (PC: −14.79%, 95% CI: −24.46%, −3.89%), while a possible positive association was seen for the upper folate group (PC: 24.36%, 95% CI: −0.09%, 54.80%). The EMM by folate groups was statistically significant (EMM p value: 0.01). No association or EMM by folate groups were found for the remaining PFAS compounds in relation to mumps antibody levels (Table 2).
Measles
No meaningful association or EMM by folate groups was observed for serum concentrations of any individual PFAS compound on measles antibody levels (Table 2).
Mixture Joint Effect
Rubella
In QGC models, the per quartile increase in serum concentrations of the PFAS mixture was significantly associated with a 7.98% (95% CI: −13.01%, −2.66%) decrease in rubella antibody levels in the total population (Table 2). Similar to the findings from the single chemical analyses, the negative joint effect of the PFAS mixture on rubella antibodies was only found in the lower folate group (PC: −9.84%, 95% CI: −15.57%, −3.74%), while not in the upper folate group (PC: −4.79%, 95% CI: −13.31%, 4.57%) (EMM p value: 0.32). BKMR confirmed the negative joint effect of the PFAS mixture and rubella antibodies in the total population and visually reported similar effect heterogeneity in the associations for the lower and upper folate groups (Figure 1). Specifically, BKMR reported a clear dose-response or linear decreasing trend of rubella antibody levels across changes by every 5th percentile of the PFAS mixture concentrations in the total population (Figure 1A) and the lower folate group (Figure 1B), while no clear trend was seen in the upper folate group (Figure 1C).
Figure 1.
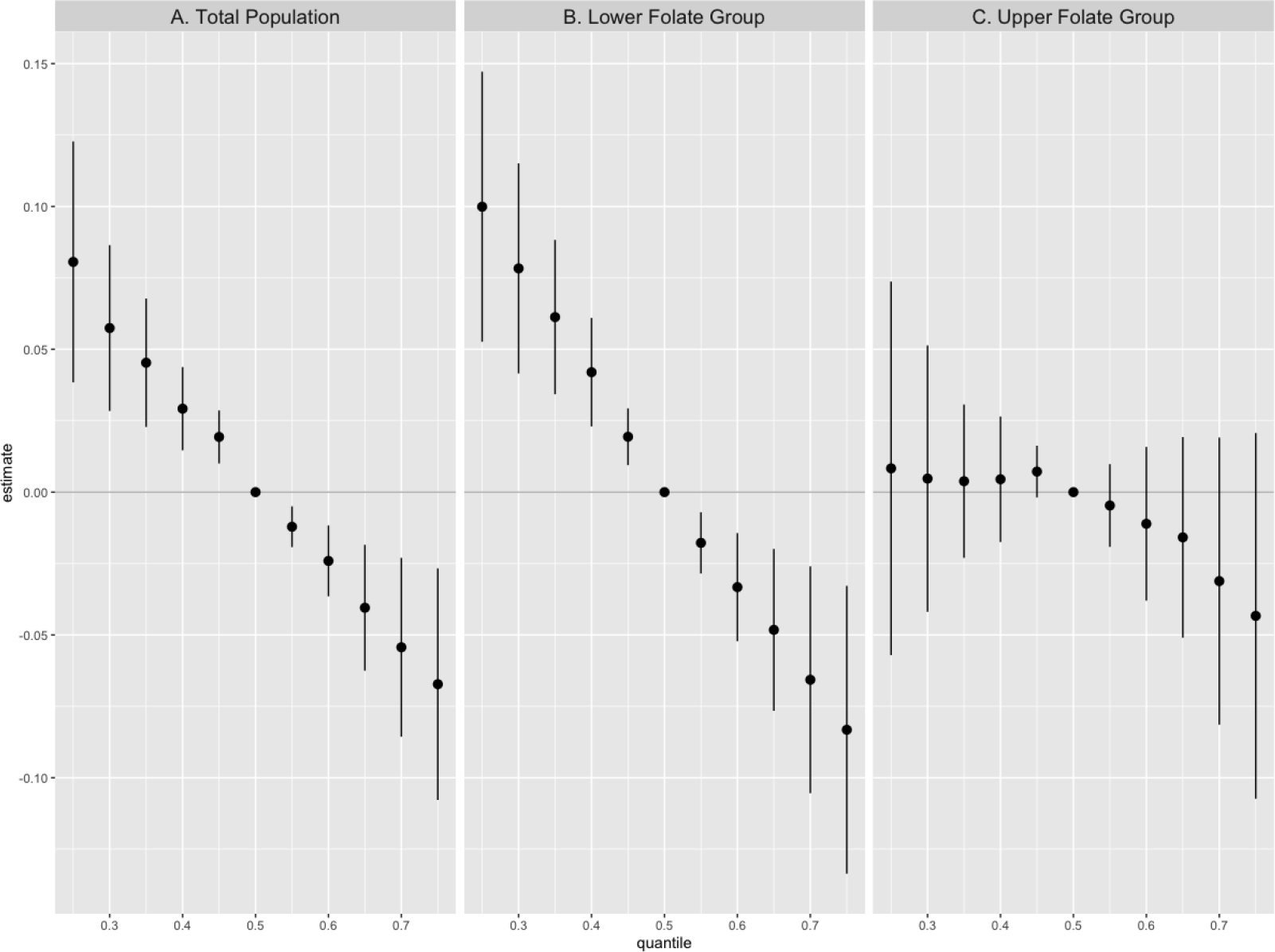
Joint effect of total serum per- and polyfluoroalkyl substances mixtures on rubella antibody levels among adolescents aged 12–19 years in the United States, results from Bayesian Kernel Machine Regression models.
Note. The point estimate shows the change in natural log-transformed rubella antibody levels per5th percentile increase or decrease from the median concentrations (reference concentrations) of the total PFAS mixtures. Models were adjusted for age (continuous), sex (dichotomous), race (categorical), income-poverty ratio (continuous), BMI (continuous), serum cotinine concentrations (continuous), survey cycle (categorical), and dietary intake of milk and milk products, eggs, and meat (continuous).
Regarding the relative contribution of individual PFAS compounds to the PFAS mixture effect on rubella antibodies, QCG reported high negative weights for PFOS in all models – total population, and lower and upper folate groups (Table S5). BKMR similarly reported a high PIP for PFOS in the model for the lower folate group, while high PIPs for PFHxS in the models of the total population and of the upper folate group (Table S6). BKMR additionally showed negative dose-response relationships between serum PFOS and PFHxS concentration and rubella antibodies in the total population and for PFOS in the lower folate group, when holding the rest of the PFAS compounds in the mixture at their median concentrations, consistent with findings from the single chemical analyses (Figure S2).
Mumps
Per quartile increase in the serum concentrations of the PFAS mixture was significantly associated with a 5.44% (95% CI: −10.44%, −0.16%) decrease in mumps antibodies in the total population (Table 2). Consistently, the joint effect of the PFAS mixture was only found in the lower folate group (PC: −8.79%, 95% CI: −14.39%, −2.82%) while not in the upper folate group (PC: 1.62%, −7.18%, 11.25%) (EMM p value: 0.04). BKMR findings were consistent with results from QGC and reported a negative joint effect of the PFAS mixture on mumps antibodies in the total population (Figure 2A) and the lower folate group (Figure 2B), while not in the upper folate group (Figure 2C).
Figure 2.
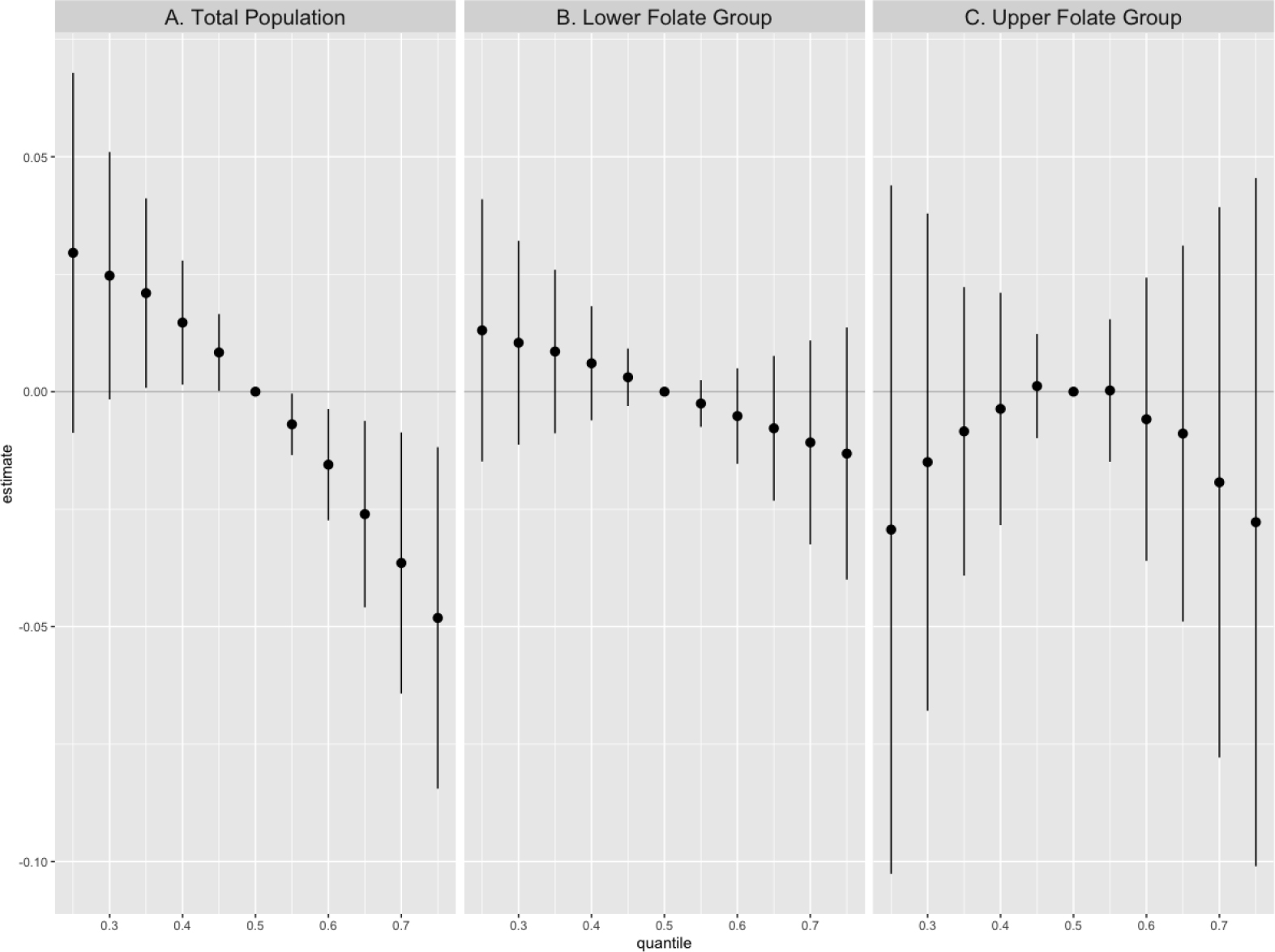
Joint effect of total serum per- and polyfluoroalkyl substances mixtures on mumps antibody levels among adolescents aged 12–19 years in the United States, results from Bayesian Kernel Machine Regression models.
Note. The point estimate shows the change in natural log-transformed mumps antibody levels per5th percentile increase or decrease from the median concentrations (reference concentrations) of the total PFAS mixtures. Models were adjusted for age (continuous), sex (dichotomous), race (categorical), income-poverty ratio (continuous), BMI (continuous), serum cotinine concentrations (continuous), survey cycle (categorical), and dietary intake of milk and milk products, eggs, and meat (continuous).
QGC showed high negative weights for PFOS in the models for the total population, lower and upper folate groups (Table S5), while BKMR showed high PIPs for PFOS and PFOA in the model for the total population, similar PIPs for the four PFAS compounds in the model for the lower folate group, and a high PIP for PFOA in the model for the upper folate group (Table S6). The dose-response relationships for individual PFAS compounds and mumps antibodies from BKMR models were imprecise with wide credible intervals and did not show any obvious trends (Figure S3).
Measles
QGC reported a positive joint effect of the PFAS mixture on measles antibodies in the total population (PC: 7.03%, 95% CI: 0.29%, 14.22%), which were imprecise in both lower (PC: 5.64%, 95% CI: −2.08%, 13.98%) and upper folate groups (PC: 9.54%, 95% CI: −1.73%, 22.11%). Similar positive joint effects of the PFAS mixture on measles antibodies were reported by BKMR models across the population groups (Figure S4). However, QGC and BKMR models reported inconsistent relative contributions of individual PFAS compounds to the mixture effect (Tables S5, S6). QGC suggested PFOS to be the most important compound (for the positive joint effect) in the total population and the lower folate group, and similar weights for PFOA and PFOS in the upper folate group (Table S5). However, BKMR reported the highest PIPs for PFOA in the total population and the upper folate group, and PFNA was the most important compound identified the lower folate group (Table S6).
Sensitivity Analyses
After redefining folate groups by the median of the RBC folate concentration, consistent EMM by folate groups as primary findings appeared in the PFOA, PFOS, and PFHxS – rubella antibody associations, and in the PFOA, PFAS mixture-mumps antibody associations. However, negative associations between PFAS mixture and rubella antibody appeared in both lower and upper folate groups (Table S7). After redefining folate groups by the lowest tertile of the RBC folate concentration, consistent EMM by folate groups was only found in the PFOA-mumps antibody association, while no EMM by folate groups was apparent in the other associations found in the primary analyses (i.e. PFOA, PFOS, PFHxS, PFAS mixture-rubella antibody associations, PFAS mixture-mumps antibody associations) (Table S8).
After stratifying the analyses by the two survey cycles, the negative (for rubella and mumps antibodies) or positive (for measles antibodies) associations between serum PFAS concentrations and antibody levels, and the EMM by folate groups found in the primary analyses remained or became stronger in the 2003–2004 cycle (Table S9). However, findings in the 2009–2010 cycle were imprecise and weaker in general, while the sample size in this cycle was smaller than the 2003–2004 cycle (298 vs. 513) (Table S9). Additionally, in the 2009–2010 cycle we found positive associations for serum PFOA, PFOS, and PFNA concentrations and mumps antibody levels in the upper folate group, which was in contrast with results from the primary analyses, although confidence intervals were wide (Table S9). Further adjustment for seafood consumption did not change the findings in the 2009–2010 cycle (Table S10).
In general, the GAM models reported that the beta coefficients of the significant PFAS-antibody associations (PFOA, PFOS, PFHxS – rubella antibody; PFOS-mumps antibody) changed in a linear fashion across RBC folate concentrations. Specifically, the beta coefficients were negative with narrower confidence intervals at lower RBC folate levels and increased into positive values with wide confidence intervals at higher RBC folate levels. The cut-off points where the beta coefficients changed from negative to null/positive was in general similar or higher than the highest tertile RBC folate level (Figures S5–8).
Discussion
In this U.S. representative sample of adolescents with seropositive antibody levels to both rubella and measles viruses, serum concentrations of PFOS, PFHxS, and the PFAS mixture were robustly associated with lower rubella antibodies in the total population and was especially apparent in the subpopulation with RBC folate concentrations below the highest tertile level (lower folate group), while not among the subpopulation whose RBC folate levels were in the top tertile (upper folate group). Similarly, we found robust associations between serum PFOA concentrations and the total PFAS mixture and lower mumps concentrations only in the lower folate group while not in the upper folate group, with evidence of statistically significant EMM by folate status. However, we reported positive associations between serum concentrations of the PFAS mixture and measles antibody levels in the total population. The findings were primarily driven by data from the 2003–2004 cycle when overall PFAS exposure was higher.
Our findings on the negative associations between PFOS and PFHxS and rubella antibody levels, and PFOA and mumps antibody levels are consistent with existing evidence, although no study assessed the joint effect of PFAS as a mixture. Only three studies examined childhood rubella antibodies in relation to prenatal or childhood PFAS exposure, of which two utilized the same NHANES data from 1999 – 2000 and 2003–2004 cycles. One study observed similar negative associations between serum PFOS and PFHxS concentrations and rubella antibodies in adolescents with seropositive rubella antibodies.9 This study is also the only one that examined PFAS exposure in relation to mumps antibody levels, finding inverse associations consistent with the current study. The other NHANES study that examined rubella antibodies in relation to PFAS exposure reported null associations among adolescents.41 Of note, this study included both seropositive and seronegative populations and defined adolescents as aged 12–18 years, which was inconsistent with the usual definition for adolescents in NHANES analyses. Including seronegative populations in the study could lead to potential unmeasured confounding, since seronegatives are very likely to be unvaccinated individuals. Only one prospective cohort examined prenatal PFAS exposure and rubella antibodies in childhood. This small Norwegian cohort of 50 mother-child pairs observed significant negative associations between prenatal serum PFAS concentrations at delivery and rubella antibodies in children at age 3, while no association was observed for measles antibodies.42 Importantly, the PFAS concentrations in this Norwegian cohort were almost three times lower than the population in the current study, suggesting that PFAS could potentially act on antibody responses even at lower exposure levels. Besides, consistent evidence is available for prenatal/childhood PFAS exposure and reduced diphtheria antibodies, with most of the evidence coming from the Faroe Islands cohort.6,7,43 There is also some evidence for PFAS exposure and reduced antibody responses to influenza,44,45 hepatitis type A and B,46 and hand-foot-and-mouth virus.47 None of the existing studies utilized mixture analyses to examine the joint effect of total PFAS mixtures on antibody levels as we did, which was important given that humans are exposed to multiple PFAS compounds simultaneously. Indeed, the most recent evaluation of the European Food Safety Authority (EFSA), considered the four PFAS compounds examined in this work together, expressing the highest concern for immunotoxicity in humans.48 We additionally reported positive associations for PFAS exposure and mumps antibody levels especially in the upper folate group in the 2009–2010 cycles. However, caution is needed in interpretating these results due to the small sample size and the wide confidence intervals of the estimates. Additionally, we did not observe associations between PFAS exposure and rubella antibody levels in the 2009–2010 cycle when the overall PFAS concentrations were lower (except for PFNA), suggesting that we could be underpowered to detect associations in this cycle with smaller sample size and lower PFAS exposure levels, although the Norwegian cohort did show associations at much lower PFAS levels.42
We found positive associations between the PFAS mixture and measles antibody levels in the total population, which was primarily driven by data from the 2003–2004 cycle. No previous study examined PFAS compounds as mixtures in relation to measles antibody levels. One NHANES study reported a null association between individual PFAS exposure and measles antibodies in adolescents,9 while two cohort studies reported null or negative associations between prenatal or early childhood PFAS exposure and measles antibodies in young children.42,49 It was unclear why we observed a positive association between the PFAS mixture and measles antibodies in the current study, while not finding associations in single-chemical analyses. Although we found this positive PFAS mixture association with measles antibodies, a lack of coherence was noticed between single-chemical and mixture analyses and between our findings and the previous literature. Together with no known biologically-plausible explanations, the most likely alternative may be residual confounding. Thus, the positive association could be possibly due to residual confounding by central variables such as socioeconomic status (SES), even though we have adjusted for major SES factors. If similar residual confounding exists in the associations for PFAS and rubella and mumps antibody levels, the true associations for these two antibodies would be even stronger than those estimated in the present study. It should also be noted that the mixture analyses could not account for the sampling scheme and weighting of the NHANES study design as were previous mixture analyses in NHANES.50–52 Thus, findings on the mixture effect may not be nationally representative, although we saw limited differences when conducting the data analyses for single-chemical models with and without accounting for the NHANES study design scheme.
This study is the first to examine folate as an effect modifier of the immune-altering actions of PFAS exposure.53 Our results support the hypothesis that the antibody-altering effect of PFAS was only present in populations with a folate status below the 66th percentile, particularly in population with higher PFAS exposure. Of note, RBC folate levels reflect medium to long-term folate intake, and at least during the previous 3 months, that is, the lifespan of red blood cells,54 suggesting that reaching optimal folate status could potentially mitigate PFAS-related immune outcomes. We additionally found that median RBC folate levels may provide “protection” for PFAS-related effects on rubella and mumps antibodies, while the lowest RBC folate level did not show the same “protective” effects on antibody levels in general, as we observed that most of the negative PFAS-antibody associations were only present in the lower folate group as defined by the median RBC folate level, but were present in both the lower and upper folate groups when defined by the lowest tertile level. Future studies are needed to further validate and explore the threshold of folate levels that can provide “protection” for PFAS-related immune outcomes in children.
Previous studies reported that folate could maintain or enhance the cytotoxicity activity of NK cells,13,55 support Th1-mediated immune responses,56 and play important roles in antibody production and metabolism.57–59 The mechanism of how folate interacts with PFAS on the immune system is currently unclear and needs further experimental exploration. There is some evidence that folate and PFAS are substrates for several shared carriers including those in the ATP-binding cassette (ABC) family 60–62 and the organic anion transporters (OAT) family.63–71 It is possible that folate could interact with PFAS on these receptors and influence their downstream effects on the immune system. Besides, as an important methyl donor, folate plays a critical role in DNA synthesis and methylation. Previous studies reported changes in DNA methylation in cord blood and in adolescent plasma in relation to prenatal PFAS exposure,72 which could be a potential pathway on how folate may mitigate PFAS-related health outcomes. Indeed, folate is able to counteract the deleterious health effects triggered by other environmental pollutants.73–75 Further, our previous study found consistent negative associations for folate status and serum PFAS concentrations in a large sample of U.S. adolescents and adults,11 and recent experimental evidence supports that spinach and soybean, which contain high levels of folate, could reduce the relative bioavailability of the absorption for PFOA and hexafluoropropylene oxide trimer acid, which is a PFOA alternative, in mice.76 Combined with findings from the current study, the existing findings implicate that an optimal medium/long-term folate intake could reduce the PFAS body burden and further counteract PFAS-related harmful effects on the immune system.
Our study innovatively examined effect modification by folate exposure on the immune-altering effects of PFAS. We were able to assess folate exposure by red blood cell folate concentration, which is probably the most reliable biomarker to estimate medium to long-term folate intake.54 The NHANES study design ensures the high quality of the data, and the representativeness and generalizability of our findings. We were also the first study to examine the joint effect of the total PFAS mixture on antibody levels. Despite these strengths, our study indeed has several limitations. First and foremost, the RBC folate concentration, serum antibodies and PFAS concentrations were measured cross-sectionally. We cannot establish causal relationships between PFAS, folate, and antibody concentrations. However, since the examined PFAS have half-lives over years and RBC folate concentration is stable within the previous three months, reverse causation where antibody levels at assessment led to different PFAS exposure within each folate group appears unlikely. Second, we used seropositivity of rubella and measles antibodies as a proxy for MMR vaccination since we did not have vaccination or boosting information in the study population, which may not be accurate, and the excluded participants could be those who had MMR vaccines but did not generate enough antibody responses. However, we would expect the number of these participants to be small since the seroconversion rates for rubella and measles were reported to be ~ 97%.18 Third, although we adjusted for a comprehensive set of covariates, residual confounding may still be possible, particularly probable in the findings for measles antibodies. Although the clinical implications by the estimated changes in rubella and mumps antibody levels associated with PFAS exposure are difficult to interpret at clinical level, additional evidence on PFAS as a risk factor for different types of infections in childhood 3,77 suggest that these associations are of public-health relevance. Lastly, while the PFAS examined in the current study are the most commonly detected and investigated long-chain PFAS compounds, NHANES data did not provide information on short-chain PFAS compounds which could also exert immunotoxic effects.78
In conclusion, in this U.S. representative study, we found inverse associations between serum concentrations of PFOS and PFHxS and the total PFAS mixture in relation to rubella antibodies, and between serum concentrations of PFOA and the total PFAS mixture in relation to mumps antibodies only in adolescents with lower red blood cell folate levels, while not among adolescents with higher folate levels. Our findings suggest that folate could potentially counteract the immune-altering effects of PFAS exposure. If confirmed in experimental settings, these findings may have important public health implications for the use of folate as a mitigation measure for PFAS-related adverse immune outcomes in children.
Supplementary Material
Synopsis:
Limited study investigated mitigation measures for PFAS’s adverse health effect. This study found the negative associations between PFAS and antibody levels were only present among children with lower folate status.
Acknowledgements
This work was supported by grants R01ES031657 and P42ES027726 from the National Institute of Environmental Health Sciences (NIEHS). We thank investigators and participants of the National Health and Nutrition Examination Study (NHANES).
Footnotes
Conflicts of Interest: The authors declare they have nothing to disclose.
Supporting Information
Additional information on the characteristics of study population, and results from mixture models and sensitivity analyses: Tables S1–10, Figures S1–4.
References
- 1.Buck RC, Franklin J, Berger U, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. Oct 2011;7(4):513–41. doi: 10.1002/ieam.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. A review of the pathways of human exposure to poly-and perfluoroalkyl substances (PFASs) and present understanding of health effects. Journal of exposure science & environmental epidemiology. 2019;29(2):131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fenton SE, Ducatman A, Boobis A, et al. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environmental Toxicology and Chemistry. 2021;40(3):606–630. doi: 10.1002/etc.4890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeWitt JC, Blossom SJ, Schaider LA. Exposure to per-fluoroalkyl and polyfluoroalkyl substances leads to immunotoxicity: epidemiological and toxicological evidence. J Expo Sci Environ Epidemiol. Mar 2019;29(2):148–156. doi: 10.1038/s41370-018-0097-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blake BE, Fenton SE. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology. Oct 2020;443:152565. doi: 10.1016/j.tox.2020.152565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grandjean P, Andersen EW, Budtz-Jørgensen E, et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. Jama. 2012;307(4):391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grandjean P, Heilmann C, Weihe P, Nielsen F, Mogensen UB, Budtz-Jørgensen E. Serum vaccine antibody concentrations in adolescents exposed to perfluorinated compounds. Environmental Health Perspectives. 2017;125(7):077018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mogensen UB, Grandjean P, Heilmann C, Nielsen F, Weihe P, Budtz-Jørgensen E. Structural equation modeling of immunotoxicity associated with exposure to perfluorinated alkylates. Environmental Health. 2015;14(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein CR, McGovern KJ, Pajak AM, Maglione PJ, Wolff MS. Perfluoroalkyl and polyfluoroalkyl substances and indicators of immune function in children aged 12–19 y: National Health and Nutrition Examination Survey. Pediatr Res. Feb 2016;79(2):348–57. doi: 10.1038/pr.2015.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwiatkowski CF, Andrews DQ, Birnbaum LS, et al. Scientific Basis for Managing PFAS as a Chemical Class. Environ Sci Technol Lett. Aug 11 2020;7(8):532–543. doi: 10.1021/acs.estlett.0c00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Mustieles V, Wang Y-X, et al. Associations of Folate Biomarkers and Serum Per- And Polyfluoroalkyl Substance (PFAS) Concentrations in U.S. Adolescents and Adults, NHANES 2003–2016. Under Review. 2022; [Google Scholar]
- 12.Zhang Y, Mustieles V, Wang Y, et al. Dietary intake and blood concentrations of folate and folic acid in relation to serum per- and polyfluoroalkyl substances (PFAS) concentrations. presented at: International Society for Environmental Epidemiology (ISEE) Annual Meeting; 2021; 10.1289/isee.2021.P-618 [DOI] [Google Scholar]
- 13.Maggini S, Pierre A, Calder PC. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients. Oct 17 2018;10(10)doi: 10.3390/nu10101531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oulhote Y, Lanphear B, Braun JM, et al. Gestational Exposures to Phthalates and Folic Acid, and Autistic Traits in Canadian Children. Environmental Health Perspectives. 2020;128(2):027004. doi: 10.1289/EHP5621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mínguez-Alarcón L, Gaskins AJ, Chiu YH, et al. Dietary folate intake and modification of the association of urinary bisphenol A concentrations with in vitro fertilization outcomes among women from a fertility clinic. Reprod Toxicol. Oct 2016;65:104–112. doi: 10.1016/j.reprotox.2016.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. Aug 7 2007;104(32):13056–61. doi: 10.1073/pnas.0703739104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention C. National Health and Nutrition Examination Survey. Accessed June 10, 2022. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm
- 18.McLean HQ, Fiebelkorn AP, Temte JL, Wallace GS. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. Jun 14 2013;62(Rr-04):1–34. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention C. Laboratory Procedures Manual. Accessed May 4, 2021. https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/2011-12_Laboratory_Procedures_Manual.pdf
- 20.Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene. 1990/01/01 1990;5(1):46–51. doi: 10.1080/1047322X.1990.10389587 [DOI] [Google Scholar]
- 21.Lubin JH, Colt JS, Camann D, et al. Epidemiologic Evaluation of Measurement Data in the Presence of Detection Limits. Environmental Health Perspectives. 2004;112(17):1691–1696. doi: 10.1289/ehp.7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention C. Laboratory Procedure Manual, Serum/Whole Blood, Microbiological Assay. Accessed May 29, 2022. https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/labmethods/FOLATE_F_met.pdf
- 23.Centers for Disease Control and Prevention C. Laboratory Procedure Manual, Bio-Rad Laboratories’ “Quantaphase II Folate/Vitamin B12” Radioassay Kit 2003–2004. Accessed May 29, 2022. https://wwwn.cdc.gov/nchs/data/nhanes/2003-2004/labmethods/l06_c_met_folates-b12.pdf
- 24.Centers for Disease Control and Prevention C. Laboratory Protocol, Solid Phase Extraction-High Performance Liquid ChromatographyTurbo Ion Spray-Tandem Mass Spectrometry (SPE-HPLC-TIS-MS/MS) Accessed May 29, 2022. https://wwwn.cdc.gov/nchs/data/nhanes/2003-2004/labmethods/l24pfc_c_met.pdf
- 25.Centers for Disease Control and Prevention C. Laboratory Procedure Manual, Online Solid Phase Extraction-High Performance Liquid Chromatography-Turbo Ion Spray-Tandem Mass Spectrometry (online SPE-HPLC-TIS-MS/MS). Accessed May 29, 2022. https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/labmethods/PFC_F_Polyfluorinated_Compounds_met.pdf
- 26.Centers for Disease Control and Prevention C. Measles, Rubella, & Varicella (L19_C). Accessed 1.3, 2022. https://wwwn.cdc.gov/Nchs/Nhanes/2003-2004/L19_C.htm
- 27.Centers for Disease Control and Prevention C. Laboratory Procedure Manual, Enzyme Immunoassay (EIA). Accessed May 29, 2022. https://wwwn.cdc.gov/nchs/data/nhanes/2003-2004/labmethods/l19_c_met_mrv.pdf
- 28.Centers for Disease Control and Prevention C. Laboratory Procedure Manual, Mumps IgG ELISA II Accessed May 29, 2022. https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/labmethods/MMRV_F_met_Mumps.pdf
- 29.Centers for Disease Control and Prevention C. Laboratory Procedure Manual, Measles IgG ELISA II Accessed May 29, 2022. https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/labmethods/MMRV_F_met_Measles.pdf
- 30.Centers for Disease Control and Prevention C. Laboratory Procedure Manual, Rubella IgG ELISA II. Accessed May 29, 2022. https://wwwn.cdc.gov/nchs/data/nhanes/2009-2010/labmethods/MMRV_F_met_Rubella.pdf
- 31.Kim S Overview of Cotinine Cutoff Values for Smoking Status Classification. Int J Environ Res Public Health. Dec 14 2016;13(12)doi: 10.3390/ijerph13121236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berg V, Nøst TH, Huber S, et al. Maternal serum concentrations of per- and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environ Int. Aug 2014;69:58–66. doi: 10.1016/j.envint.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 33.Eick SM, Goin DE, Trowbridge J, et al. Dietary predictors of prenatal per- and poly-fluoroalkyl substances exposure. J Expo Sci Environ Epidemiol. Oct 6 2021;doi: 10.1038/s41370-021-00386-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lasters R, Groffen T, Eens M, et al. Home-produced eggs: An important human exposure pathway of perfluoroalkylated substances (PFAS). Chemosphere. Dec 2022;308(Pt 1):136283. doi: 10.1016/j.chemosphere.2022.136283 [DOI] [PubMed] [Google Scholar]
- 35.Ulven SM, Holven KB, Gil A, Rangel-Huerta OD. Milk and Dairy Product Consumption and Inflammatory Biomarkers: An Updated Systematic Review of Randomized Clinical Trials. Adv Nutr. May 1 2019;10(suppl_2):S239–s250. doi: 10.1093/advances/nmy072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Childs CE, Calder PC, Miles EA. Diet and Immune Function. Nutrients. Aug 16 2019;11(8)doi: 10.3390/nu11081933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environmental Health Perspectives. 2020;128(4):047004. doi: 10.1289/EHP5838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bobb JF, Valeri L, Claus Henn B, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. Jul 2015;16(3):493–508. doi: 10.1093/biostatistics/kxu058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seshasayee SM, Rifas-Shiman SL, Chavarro JE, et al. Dietary patterns and PFAS plasma concentrations in childhood: Project Viva, USA. Environ Int. Jun 2021;151:106415. doi: 10.1016/j.envint.2021.106415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendivil CO. Dietary Fish, Fish Nutrients, and Immune Function: A Review. Front Nutr. 2020;7:617652. doi: 10.3389/fnut.2020.617652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilkerton CS, Hobbs GR, Lilly C, Knox SS. Rubella immunity and serum perfluoroalkyl substances: Sex and analytic strategy. PLOS ONE. 2018;13(9):e0203330. doi: 10.1371/journal.pone.0203330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granum B, Haug LS, Namork E, et al. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol. Oct-Dec 2013;10(4):373–9. doi: 10.3109/1547691x.2012.755580 [DOI] [PubMed] [Google Scholar]
- 43.Kielsen K, Shamim Z, Ryder LP, et al. Antibody response to booster vaccination with tetanus and diphtheria in adults exposed to perfluorinated alkylates. J Immunotoxicol. 2016;13(2):270–3. doi: 10.3109/1547691x.2015.1067259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein CR, Ge Y, Wolff MS, et al. Perfluoroalkyl substance serum concentrations and immune response to FluMist vaccination among healthy adults. Environ Res. Aug 2016;149:171–178. doi: 10.1016/j.envres.2016.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Looker C, Luster MI, Calafat AM, et al. Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicol Sci. Mar 2014;138(1):76–88. doi: 10.1093/toxsci/kft269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shih YH, Blomberg AJ, Bind MA, et al. Serum vaccine antibody concentrations in adults exposed to per- and polyfluoroalkyl substances: A birth cohort in the Faroe Islands. J Immunotoxicol. Dec 2021;18(1):85–92. doi: 10.1080/1547691x.2021.1922957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng XW, Bloom MS, Dharmage SC, et al. Prenatal exposure to perfluoroalkyl substances is associated with lower hand, foot and mouth disease viruses antibody response in infancy: Findings from the Guangzhou Birth Cohort Study. Sci Total Environ. May 1 2019;663:60–67. doi: 10.1016/j.scitotenv.2019.01.325 [DOI] [PubMed] [Google Scholar]
- 48.Schrenk D, Bignami M, Bodin L, et al. Risk to human health related to the presence of perfluoroalkyl substances in food. Efsa j. Sep 2020;18(9):e06223. doi: 10.2903/j.efsa.2020.6223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Timmermann CAG, Jensen KJ, Nielsen F, et al. Serum Perfluoroalkyl Substances, Vaccine Responses, and Morbidity in a Cohort of Guinea-Bissau Children. Environmental health perspectives. 2020;128(8):87002–87002. doi: 10.1289/EHP6517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Dong T, Hu W, et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ Int. Feb 2019;123:325–336. doi: 10.1016/j.envint.2018.11.076 [DOI] [PubMed] [Google Scholar]
- 51.Bulka CM, Avula V, Fry RC. Associations of exposure to perfluoroalkyl substances individually and in mixtures with persistent infections: Recent findings from NHANES 1999–2016. Environ Pollut. Apr 15 2021;275:116619. doi: 10.1016/j.envpol.2021.116619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, Mustieles V, Sun Y, Oulhote Y, Wang YX, Messerlian C. Association between serum per- and polyfluoroalkyl substances concentrations and common cold among children and adolescents in the United States. Environ Int. Jun 2022;164:107239. doi: 10.1016/j.envint.2022.107239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roth K, Imran Z, Liu W, Petriello MC. Diet as an Exposure Source and Mediator of Per- and Polyfluoroalkyl Substance (PFAS) Toxicity. Front Toxicol. 2020;2:601149. doi: 10.3389/ftox.2020.601149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bailey LB, Stover PJ, McNulty H, et al. Biomarkers of Nutrition for Development-Folate Review. J Nutr. Jul 2015;145(7):1636s–1680s. doi: 10.3945/jn.114.206599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maggini S, Beveridge S, Sorbara P, Senatore G. Feeding the immune system: the role of micronutrients in restoring resistance to infections. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources. 2008;3(098):1–21. [Google Scholar]
- 56.Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab. 2007;51(4):301–23. doi: 10.1159/000107673 [DOI] [PubMed] [Google Scholar]
- 57.Saeed F, Nadeem M, Ahmed RS, Tahir Nadeem M, Arshad MS, Ullah A. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds – a review. Food and Agricultural Immunology. 2016/03/03 2016;27(2):205–229. doi: 10.1080/09540105.2015.1079600 [DOI] [Google Scholar]
- 58.Maggini S, Wintergerst ES, Beveridge S, Hornig DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. Oct 2007;98 Suppl 1:S29–35. doi: 10.1017/s0007114507832971 [DOI] [PubMed] [Google Scholar]
- 59.Gombart AF, Pierre A, Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients. Jan 16 2020;12(1)doi: 10.3390/nu12010236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dankers AC, Roelofs MJ, Piersma AH, et al. Endocrine disruptors differentially target ATP-binding cassette transporters in the blood-testis barrier and affect Leydig cell testosterone secretion in vitro. toxicological sciences. 2013;136(2):382–391. [DOI] [PubMed] [Google Scholar]
- 61.Cannon RE, Richards AC, Trexler AW, et al. Effect of GenX on P-Glycoprotein, Breast Cancer Resistance Protein, and Multidrug Resistance–Associated Protein 2 at the Blood–Brain Barrier. Environmental health perspectives. 2020;128(3):037002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X, Cheng X, Lei B, Zhang G, Bi Y, Yu Y. A review of the transplacental transfer of persistent halogenated organic pollutants: Transfer characteristics, influential factors, and mechanisms. Environ Int. Jan 2021;146:106224. doi: 10.1016/j.envint.2020.106224 [DOI] [PubMed] [Google Scholar]
- 63.Shulpekova Y, Nechaev V, Kardasheva S, et al. The Concept of Folic Acid in Health and Disease. Molecules. 2021;26(12):3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kummu M, Sieppi E, Koponen J, et al. Organic anion transporter 4 (OAT 4) modifies placental transfer of perfluorinated alkyl acids PFOS and PFOA in human placental ex vivo perfusion system. Placenta. Oct 2015;36(10):1185–91. doi: 10.1016/j.placenta.2015.07.119 [DOI] [PubMed] [Google Scholar]
- 65.Matherly LH, Goldman DI. Membrane transport of folates. Vitam Horm. 2003;66:403–56. doi: 10.1016/s0083-6729(03)01012-4 [DOI] [PubMed] [Google Scholar]
- 66.Ducatman A, Luster M, Fletcher T. Perfluoroalkyl substance excretion: Effects of organic anion-inhibiting and resin-binding drugs in a community setting. Environ Toxicol Pharmacol. Jul 2021;85:103650. doi: 10.1016/j.etap.2021.103650 [DOI] [PubMed] [Google Scholar]
- 67.Worley RR, Yang X, Fisher J. Physiologically based pharmacokinetic modeling of human exposure to perfluorooctanoic acid suggests historical non drinking-water exposures are important for predicting current serum concentrations. Toxicol Appl Pharmacol. Sep 1 2017;330:9–21. doi: 10.1016/j.taap.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang CH, Glover KP, Han X. Characterization of cellular uptake of perfluorooctanoate via organic anion-transporting polypeptide 1A2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicol Sci. Oct 2010;117(2):294–302. doi: 10.1093/toxsci/kfq219 [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Beesoon S, Zhu L, Martin JW. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol. Sep 17 2013;47(18):10619–27. doi: 10.1021/es401905e [DOI] [PubMed] [Google Scholar]
- 70.Bangma J, Guillette TC, Bommarito PA, et al. Understanding the dynamics of physiological changes, protein expression, and PFAS in wildlife. Environ Int. Jan 15 2022;159:107037. doi: 10.1016/j.envint.2021.107037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma D, Lu Y, Liang Y, et al. A Critical Review on Transplacental Transfer of Per- and Polyfluoroalkyl Substances: Prenatal Exposure Levels, Characteristics, and Mechanisms. Environ Sci Technol. May 17 2022;56(10):6014–6026. doi: 10.1021/acs.est.1c01057 [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, Eliot MN, Papandonatos GD, et al. Gestational Perfluoroalkyl Substance Exposure and DNA Methylation at Birth and 12 Years of Age: A Longitudinal Epigenome-Wide Association Study. Environmental Health Perspectives. 2022;130(3):037005. doi: 10.1289/EHP10118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proceedings of the National Academy of Sciences. 2007;104(32):13056–13061. doi: 10.1073/pnas.0703739104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bragg M, Chavarro JE, Hamra GB, et al. Prenatal Diet as a Modifier of Environmental Risk Factors for Autism and Related Neurodevelopmental Outcomes. Current Environmental Health Reports. 2022/06/01 2022;9(2):324–338. doi: 10.1007/s40572-022-00347-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mínguez-Alarcón L, Gaskins AJ, Chiu Y-H, et al. Dietary folate intake and modification of the association of urinary bisphenol A concentrations with in vitro fertilization outcomes among women from a fertility clinic. Reproductive Toxicology. 2016/10/01/ 2016;65:104–112. doi: 10.1016/j.reprotox.2016.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cui X, Gu Q, Juhasz A, Chen Y. In vivo relative bioavailability of perfluorooctanoic acid (PFOA) and its alternative hexafluoropropylene oxide trimer acid (HFPO-TA): Influence of food and mechanisms exploration. Environment International. 2022/10/01/ 2022;168:107450. doi: 10.1016/j.envint.2022.107450 [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y, Mustieles V, Sun Y, Oulhote Y, Wang Y-X, Messerlian C. Association between serum per- and polyfluoroalkyl substances concentrations and common cold among children and adolescents in the United States. Environment International. 2022/06/01/ 2022;164:107239. doi: 10.1016/j.envint.2022.107239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Woodlief T, Vance S, Hu Q, DeWitt J. Immunotoxicity of Per- and Polyfluoroalkyl Substances: Insights into Short-Chain PFAS Exposure. Toxics. May 1 2021;9(5)doi: 10.3390/toxics9050100 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


