Abstract
Floating aortic arch thrombi—blood clots forming in an aorta without aneurysms or atherosclerosis—in a normal aorta are exceedingly rare. The etiology is unknown, and there are no guidelines for appropriate treatment strategies. We report a case of floating aortic arch thrombosis in a patient without coagulopathy that was treated surgically. As the mass could not be identified preoperatively as a tumor or thrombus, synthetic graft replacement was performed, allowing resection of the lesion site. Histopathological examination revealed erosion and fissures in the tunica intima of the aorta, which suggested vessel damage to the tunica intima as the cause.
Keywords: floating aortic arch thrombus, aorta tunica intima, CD34-positive endothelial progenitor cells
Introduction
Aortic mural thrombus in a non-aneurysmal or non-atherosclerotic aorta is rare. Early surgery is recommended due to the high risk of recurrence and systemic embolism, especially in patients with thrombi in the ascending aorta or aortic arch.1) Surgical procedures include thrombectomy, endarterectomy, graft replacement, thrombus aspiration, and stent grafting. The mechanism behind the formation of aortic mural thrombi without aneurysm or atherosclerosis remains unclear. Some reports suggest congenital/acquired thrombophilia or tumor to be the cause, while other reports do not.2)
We herein report a case of aortic arch mural thrombosis in a patient without coagulopathy that was successfully treated surgically.
Case Report
A 72-year-old woman was hospitalized with cholangitis. She had a history of hypertension, dyslipidemia, and diabetes mellitus, but no history of smoking or any medical history of hematologic disease, thrombophilia, tumors, or trauma. Contrast-enhanced computed tomography (CT) incidentally revealed an asymptomatic mass in the aortic arch that was not hostile and mild atheosclerosis (Figs. 1A and 1B). Although it was difficult to visually differentiate if the mass was a thrombus or a tumor, anticoagulation therapy with heparin was initiated to address possible blood clots. Laboratory blood analysis, such as C3, C4, 50% hemolytic complement, antithrombin III, protein S/C, anti-cardiolipin β2-glycoprotein I, myeloperoxidase-antineutrophil cytoplasmic antibodies, proteinase 3-antineutrophil cytoplasmic antibodies, lupus anticoagulant, homocysteine, and tumor markers, showed no predisposition to thrombosis.
Fig. 1 Contrast-enhanced computed tomography (CT) and transesophageal echocardiography image findings. (A, B) Preoperative contrast-enhanced CT scan shows a 16×12 mm mass in the aortic arch. (C, D) Transesophageal echocardiography shows an approximately 15 mm mobile mass in the aortic arch.
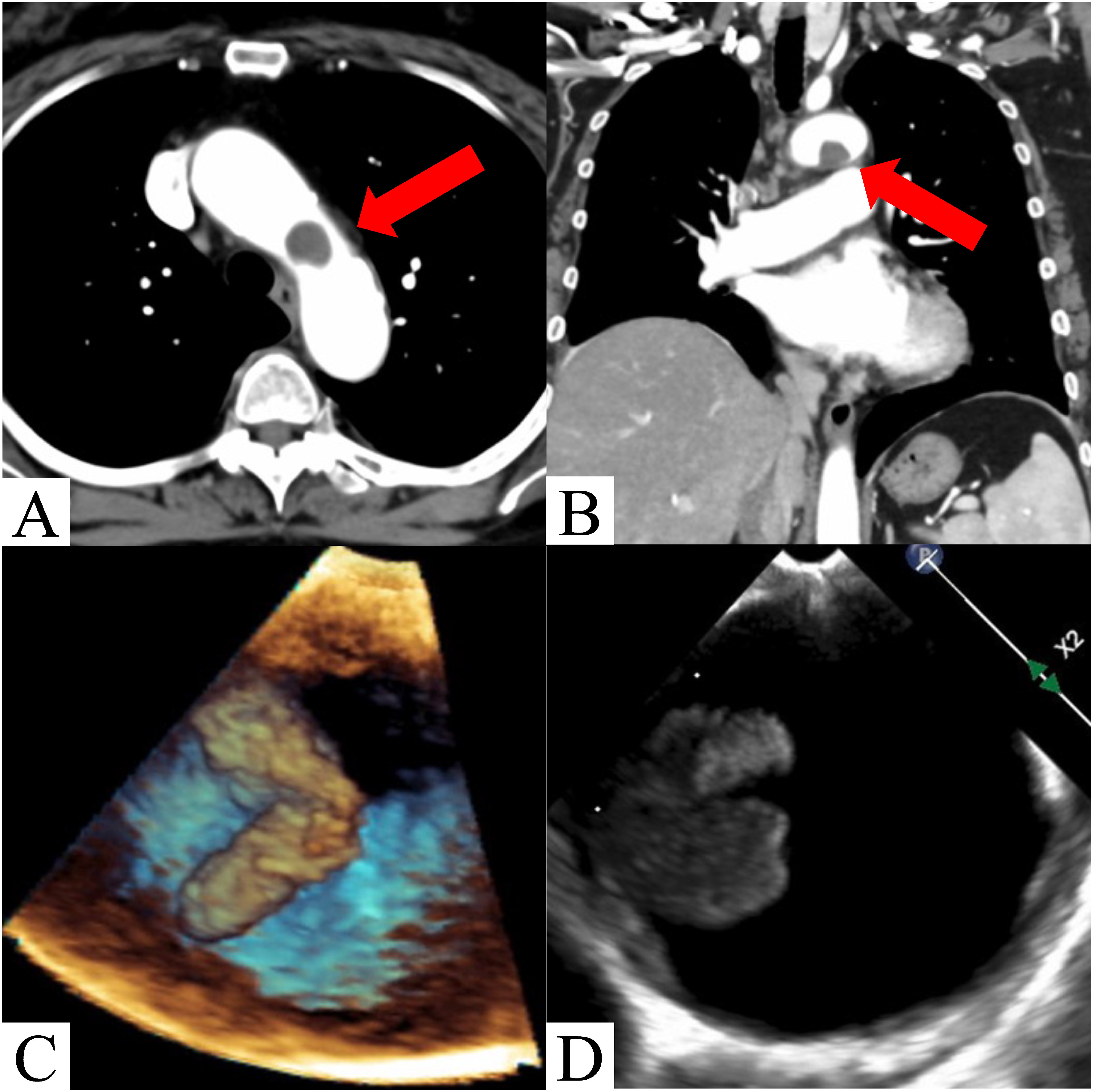
Transesophageal echocardiography, performed 1 week later, revealed that the mass in the aortic arch was pedunculated and mobile (Figs. 1C and 1D). As the mass was mobile and there was no significant improvement following anticoagulation therapy, we performed surgery the next day after considering the risk of embolism.
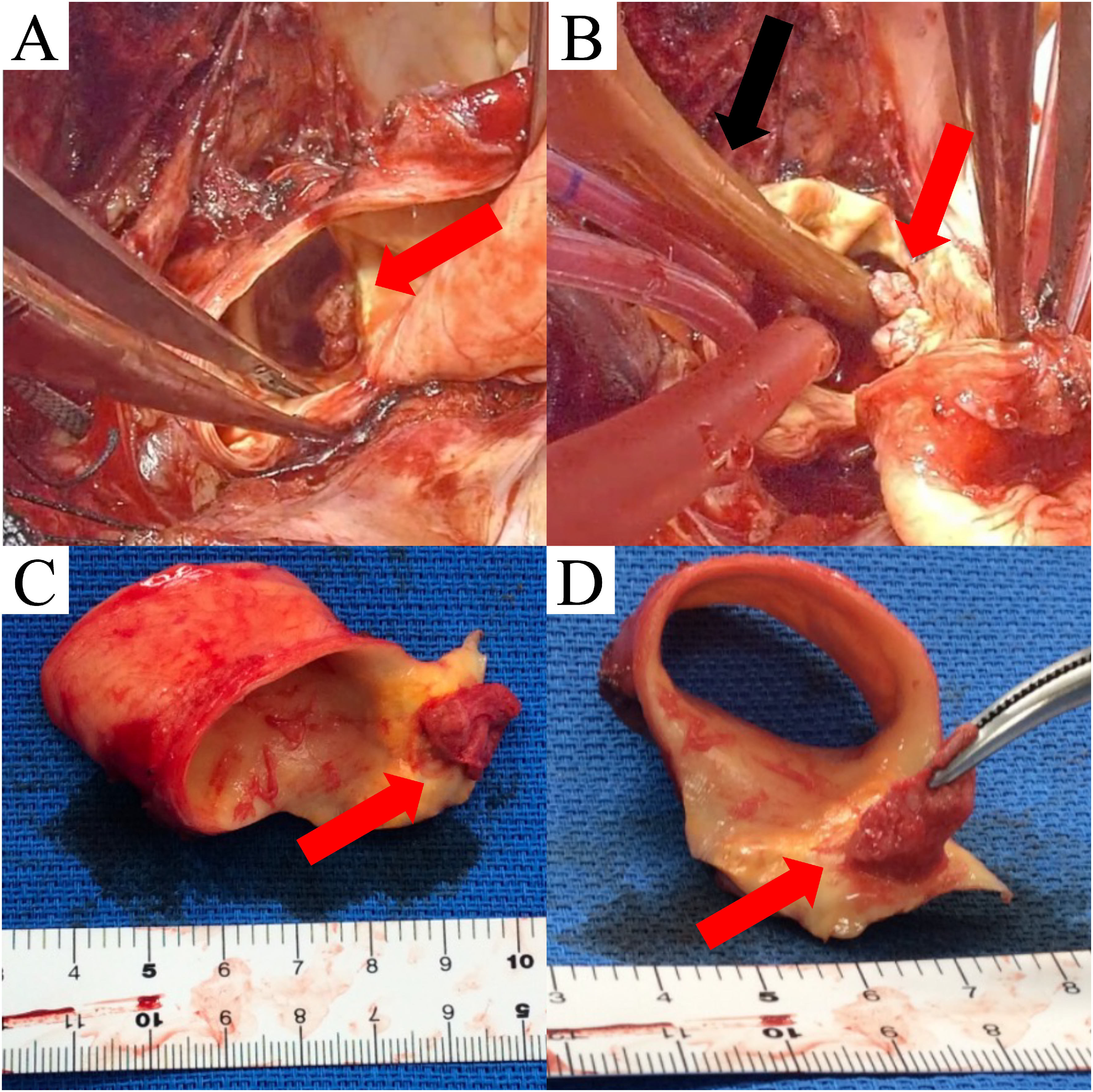
Surgery was performed under general anesthesia via median sternotomy. After systemic heparinization, cardiopulmonary bypass was established by single right atrial venous drainage and arterial perfusion was achieved via cannulation of the ascending aorta (Duraflo FEM II 20Fr, Edwards Lifesciences, Irvine, CA, USA). The rectal temperature subsequently decreased to 25°C. Hypothermic circulatory arrest and antegrade selective cerebral perfusion were also performed. The mass was located on the lesser curvature of the distal aortic arch (Figs. 2A and 2B). The aortic wall was resected between the ascending aorta and distal aortic arch through the lesser curvature of the aortic arch to extract the mass with adequate margins (Figs. 2C and 2D). The peninsula of the aortic arch branches was spared and the continuity of the greater curvature side of the distal arch was retained. The distal end of a synthetic graft (J Graft 1 Branched 28 mm, Japan Lifeline, Tokyo, Japan) was then trimmed with a diagonal cut to fit the native distal aortic orifice. The anastomosis was performed via a single continuous 4-0 polypropylene suture without the need for a felt strip. An intraoperative histopathological rapid diagnosis of the mass indicated an aortic wall thrombus. The operation time was 257 min, the cardiopulmonary bypass time was 128 min, the aortic cross-clamp time was 41 min, the selective cerebral perfusion time was 49 min, and the minimum rectal temperature was 27°C.
Fig. 2 Intraoperative findings. (A, B) The red arrows show that the mass is located on the lesser curvature side of the aortic arch. The black arrow shows a 14 Fr Foley catheter inserted to prevent the mass from falling to the peripheral side. (C, D) The resected aortic wall and mass.
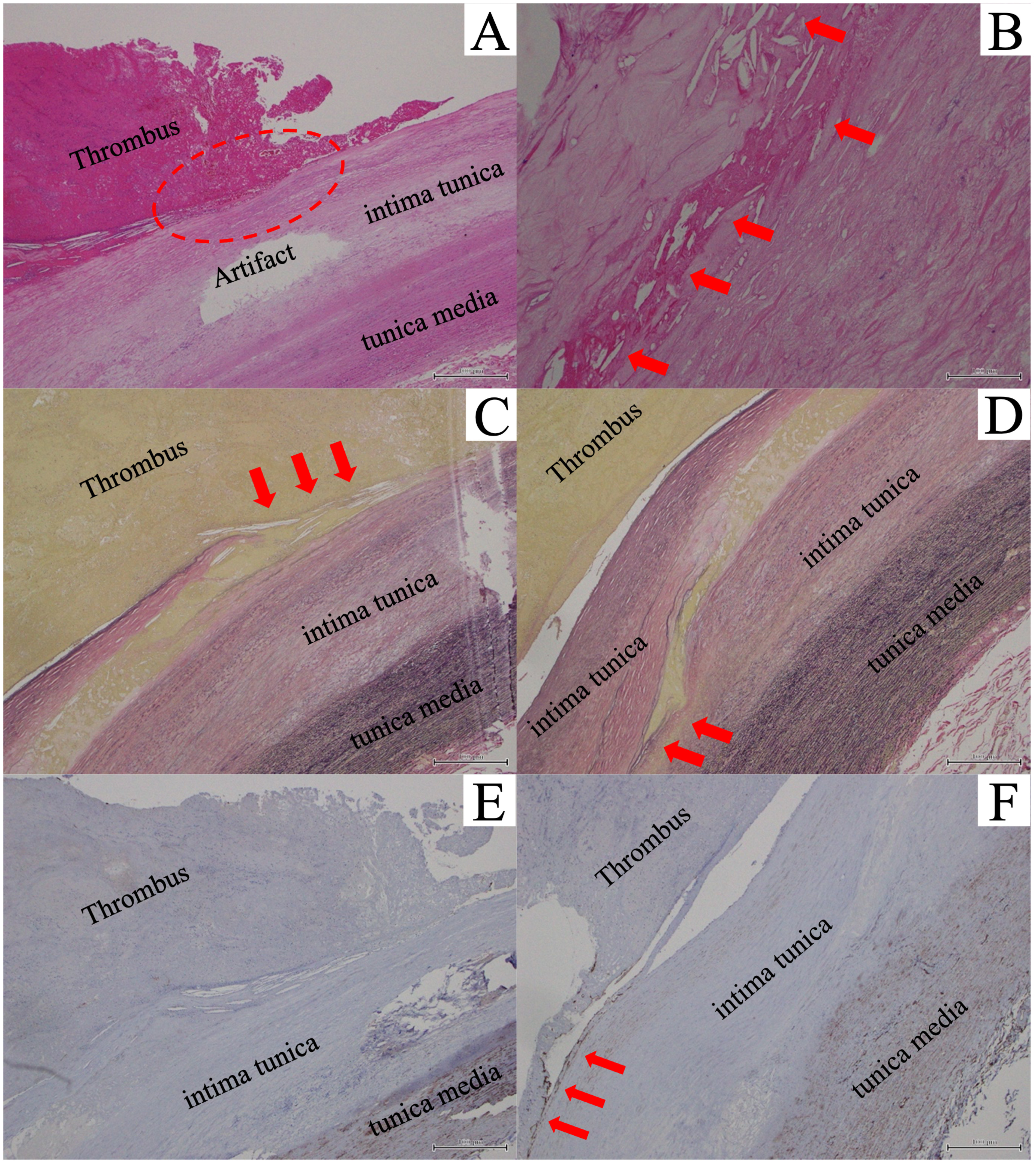
Postoperatively, the patient was admitted to the intensive care unit and discharged the next day. Aspirin and vitamin K antagonists were administered. Postoperative three-dimensional CT images showed no recurrence of the aortic arch thrombus, and her postoperative course was uneventful. A histopathological examination revealed no evidence of malignancy in the mass and resected arterial wall. Although the aorta appeared normal in preoperative imaging, histopathology showed the presence of intimal erosion and cholesterin crystals with atherosclerosis (Figs. 3A and 3B). Moreover, part of the intimal erosion had ruptured, filling the area with blood and forming a thrombus (Figs. 3C and 3D). Immunohistochemistry revealed CD34+ endothelial progenitor cells (EPCs), present only around the intimal defect (Figs. 3E and 3F).
Fig. 3 Pathological findings. (A, B) Hematoxylin and eosin stain of the resected aortic arch with thrombus: (A) Red clots contain red blood cells, fibrin, and white blood cells. The red circled area shows a finding of intimal erosion. (B) The red arrows show cholesterin crystals, which suggest atherosclerosis (A, 40× magnification; B, 400× magnification). (C, D) Elastica van Gieson stain of the resected aortic arch with thrombus: (C) The red arrows show the discontinuity of the intima, which is filled with blood components. (D) The red arrows show the tip of the fissure. The elastic fibers of the tunica media retain their structure, suggesting that the damage is confined to the intima tunica only (C and D, 100× magnification). (E, F) CD34 immunostaining. (E) No positive CD34 staining of cells in the vascular intima. (F) The red arrows show CD34-positive endothelial progenitor cells (CD34+ endothelial progenitor cells) only on the edge of the thrombus (E, 40× magnification; F, 100× magnification).
Discussion
Aortic mural thrombi without aneurysm or atherosclerosis are rare. Cases of floating thrombi in the aortic arch have been reported even less frequently. Consequently, there are no guidelines for treatment strategies, although there are various reports on anticoagulation and surgical treatments. A meta-analysis by Fayad et al.1) demonstrated that only anticoagulation treatment is associated with high rates of embolism, recurrence, and limb amputation, especially in patients with thrombi present in the ascending or arching aorta. Hence, prompt administration of heparin and early surgical intervention in low-risk patients were recommended.1) Surgical procedures include thrombectomy, endarterectomy, graft replacement, thrombus aspiration, and stent grafting.3–7) In this case, transesophageal echocardiography showed markedly high mobility, which, despite the absence of embolism, indicated the need for urgent surgery. Whether the mass was a tumor or a thrombus was unclear, and there was also a possible abnormality in the aorta’s intima itself. Therefore, we proceeded with replacement using a synthetic graft after considering the chances of recurrence and systemic embolism and the low risk associated with the surgery. Magnetic resonance imaging, gallium scintigraphy, and positron emission tomography-CT would have been desirable for differentiation; however, there was no time to perform these tests.
The mass was located on the lesser curvature of the distal aortic arch. Therefore, total arch replacement was an optional procedure for aortic recontraction. However, we only replaced the partial aortic arch to spare as much of the normal and/or healthy aortic wall as possible. In addition, it allowed for a shorter operation time because of not needing to perform aortic arch branch recontractions separately. Thus, the partial arch replacement method may be useful in cases where recontraction of the aortic arch branches is unnecessary, including in type A aortic dissection.
In patients with mobile, floating thrombus, or flail atheroma in the aortic arch, the risk of embolism due to hydrodynamic injury, also known as the “sand-blast effect,” from the aortic perfusion cannula must be considered.8) The mass was located on the lesser curvature of the distal arch, and the risk of cerebral infarction from arterial cannulation in the ascending aorta was considered low. Retrograde blood delivery from the femoral artery posed a high risk of cerebral infarction, and blood delivery from the axillary artery was complicated and time consuming due to the small vessel diameter and obesity. Therefore, the usual method of cannulation was performed from the ascending aorta.
Thrombogenic predispositions, such as protein S deficiency, protein C deficiency, antithrombin III deficiency, antiphospholipid antibodies, trauma, and malignancy, have been reported as causes of aortic thrombus.9) However, patients without thrombogenic factors have also been reported.2) Therefore, the mechanism of thrombus formation cannot be solely explained by the presence of a thrombotic predisposition. When considering thrombus formation, Virchow’s triad, namely, hypercoagulability, stagnation of blood flow, and vessel wall injury, is widely recognized as a major factor promoting thrombus formation. In this case, we presumed that vessel wall injury was involved as there was no blood stasis in the fast-flowing aorta, nor did the patient have any thrombotic predisposition.
Few reports have examined the pathological etiology of this disease.3–5) In these, intimal erosion was identified as the cause.4,5) In the present case, the fissure was caused by the fragility of the intima tunica against the background of intimal erosion with atherosclerosis. Unlike in aortic dissection, the structure of the tunica media was preserved. The fissure was filled with blood components, and thrombosis of the fissure may have reduced the pressure on the tip of the fissure and prevented further progression.
CD34+ EPCs gather at sites of vascular injury and participate in vascular endothelial repair and thrombus formation9); these cells have also been observed in aortic floating thrombus.4,5) Indeed, in mouse and rabbit models of vascular injury, CD34+ EPCs accumulate at the site of injury several weeks after the injury.10) In contrast to previous reports, in our case, histopathology showed that CD34+ EPCs were only partially present around the intimal defect,5) possibly due to early incidental discovery of the thrombus after intimal laceration. Thus, this may represent an early stage before CD34+ EPCs are recruited to the thrombus site.
Although the cause and mechanism of aortic mural thrombosis without atherosclerosis or aneurysm remain unknown, histopathology results suggested that intimal erosion and fissures may have caused thrombus formation. Evidence of the abnormality in the intima tunica itself may explain the high recurrence rate associated with anticoagulation therapy.
Conclusion
Surgical treatment for floating aortic arch thrombosis without coagulopathy resulted in good clinical results. The aortic arch thrombosis without atherosclerosis or aneurysms was caused by an intimal erosion too fine to be detected by imaging methods, such as CT scans or transesophageal echocardiography. It is possible to estimate whether an early onset condition is present by examining the CD34+ EPC recruitment.
Acknowledgments
None
Informed Consent and Ethics Statement
Written informed consent was obtained from the patient. IRB number: RIN-859. This study was approved by the Research Ethics Committee, Juntendo University Shizuoka Hospital (May 20, 2022).
Disclosure Statement
All authors declare no conflict of interest.
Author Contributions
Study conception: RO, KK, WF, HW, MO
Data collection: RO, WF, YN, YS
Analysis: RO, RW
Investigation: RO
Manuscript preparation: RO, MO, KK
Funding acquisition: None
Critical review and revision: all authors
Final approval of the article: all authors
Accountability for all aspects of the work: all authors
Supplementary Information
Supplementary movie is available at the online article sites on J-STAGE and PMC.
Supplementary Data
References
- 1).Fayad ZY, Semaan E, Fahoum B, et al. Aortic mural thrombus in the normal or minimally atherosclerotic aorta. Ann Vasc Surg 2013; 27: 282-90. [DOI] [PubMed] [Google Scholar]
- 2).Daniel PC, Mónica GB, Jorge MM, et al. Surgical approach for a symptomatic aortic arch thrombus. Interact Cardiovasc Thorac Surg 2017; 24: 288-99. [DOI] [PubMed] [Google Scholar]
- 3).Kawaguchi S, Miyano Y, Goto S, et al. A mobile thrombus in the aortic arch. Jpn J Cardiovasc Surg 2021; 50: 57-60. [Google Scholar]
- 4).Kawai Y, Koizumi K, Itoh T, et al. Mobile thrombus in the ascending aorta. Ann Vasc Dis 2020; 13: 69-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Nishizaki F, Tomita H, Abe N, et al. Acute myocardial infarction caused by a floating thrombus in the ascending aorta: a role of CD34-positive endothelial cells. J Cardiol Cases 2013; 8: e88-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Verma H, Meda N, Vora S, et al. Contemporary management of symptomatic primary aortic mural thrombus. J Vasc Surg 2014; 60: 1524-34. [DOI] [PubMed] [Google Scholar]
- 7).Qintar M, Wang DD, O’Neill WW, et al. Vacuum to the rescue: aspiration of a large mobile aortic arch thrombus with the AngioVac system utilizing transcaval access. J Invasive Cardiol 2021; 33: E756-7. [DOI] [PubMed] [Google Scholar]
- 8).Fukuda I, Daitoku K, Minakawa M, et al. Shaggy and calcified aorta: surgical implications. Gen Thorac Cardiovasc Surg 2013; 61: 301-13. [DOI] [PubMed] [Google Scholar]
- 9).Sanon S, Phung MK, Lentz R, et al. Floating, non-occlusive, mobile aortic thrombus and splenic infarction associated with protein C deficiency. J Am Soc Echocardiogr 2009; 22: 1419.e1-3. [DOI] [PubMed] [Google Scholar]
- 10).Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


