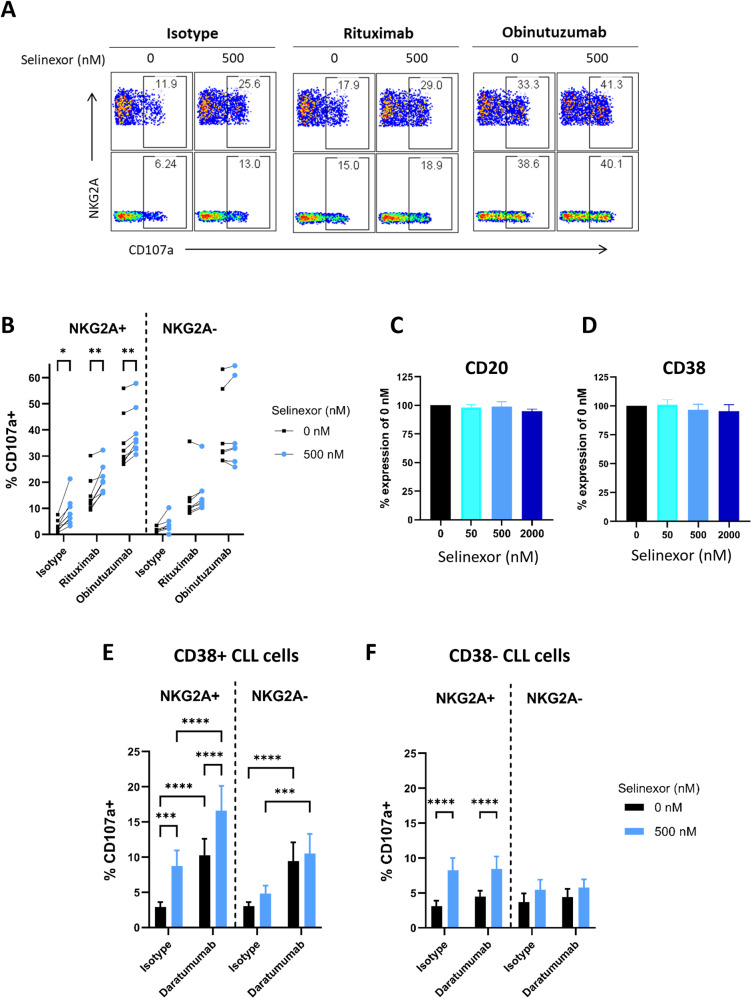
Fig. 5. Selinexor enhances NK cell activation against CLL cells in combination with anti-CD20 and anti-CD38 mAb.
Flow cytometry plots of NKG2A+ and NKG2A- NK cell activation measured by CD107a expression against CLL cells pre-treated with selinexor and anti-CD20 mAbs rituximab and obinutuzumab or isotype control (A). CLL cells were treated with 500 nM selinexor or DMSO for 24 h and incubated with 0.1 µg/mL anti-CD20 mAb rituximab or obinutuzumab or isotype control for 20 min prior to co-culture with healthy donor PBMC (E:T = 1:1). NK cell activation in NKG2A+ and NKG2A- NK cells was measured by CD107a staining minus the no target control for each mAb (B). Each line in (B) represents matched co-cultures for each antibody condition (N = 8). Significant differences in NK cell activation between selinexor concentrations were assessed by repeated-measure two-way ANOVA followed by Sidak’s multiple comparison test (*P < 0.05, **P < 0.01). C, D Expression of CD20 (B, N = 5) and CD38 (C, N = 4) on CD5 + CD19 + CLL cells after treatment with selinexor (50–2000 nM) for 24 h. E, F NK cell activation in NKG2A+ and NKG2A- NK cells against selinexor-treated CLL cells in the presence of daratumumab (anti-CD38). CLL cells derived from donors positive (D, N = 7) and negative (E, N = 11) for CD38 were treated with 500 nM selinexor for 24 h and co-cultured with healthy donor PBMC the next day (E:T = 1:1). Prior to co-culture CLL cells were incubated with daratumumab or isotype control for 20 min at a concentration of 0.1 µg/mL. Shown is the mean ± SEM and differences in NK cell activation between treatments were assessed by repeated-measure two-way ANOVA followed by Tukey’s post-hoc test (***P < 0.005, ****P < 0.001).