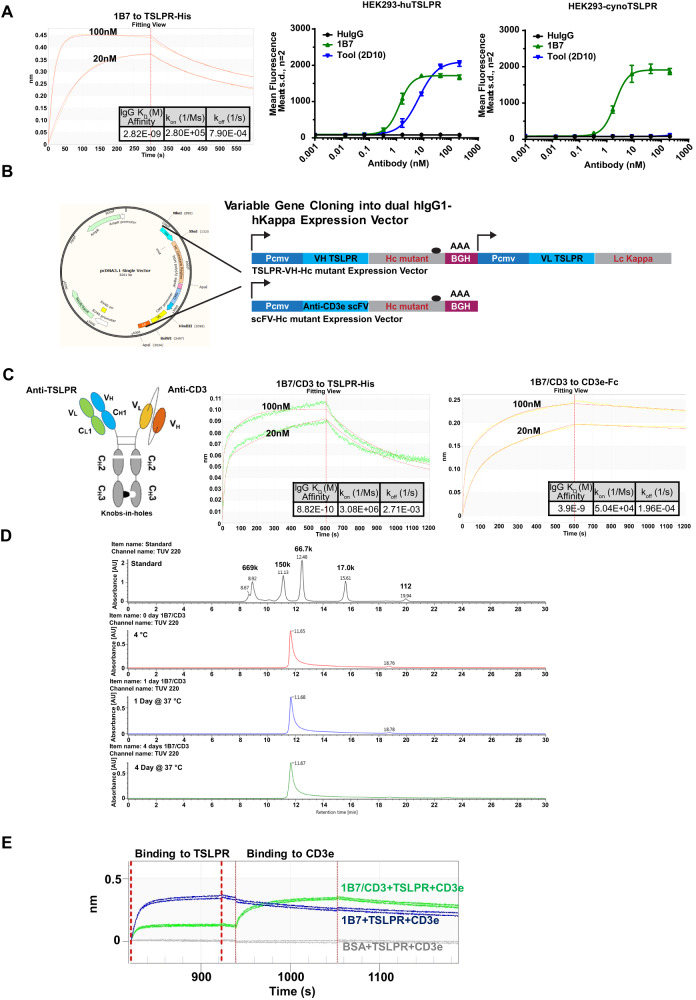
Fig. 1. Development and characteristics of the 1B7/CD3 bispecific antibody (BsAb).
A Octet and cell surface binding of the top clone 1B7 with TSLPR-His (lab prepared monomer structure confirmed), human TSLPR (huTSLPR, and cynomolgus monkey TSLPR (cynoTSLPR) (B) Design of both TSLPR-VH-Hc mutant and anti-CD3e-VH-Hc mutant (scFv) vectors in pcDNA3.1 for bispecific monoclonal antibody expression. C Left, cartoon of the 1B7/CD3 BsAb with a non-functional Fc region; Middle and right panel, 1B7/CD3 BsAb was tested for binding to either antigen TSLPR or CD3e. D The stability of 1B7/CD3 BsAb was tested by ultra-high performance liquid chromatography-size exclusion chromatography (UHPLC-SEC) at indicated time points and temperatures. E Tandem binding of 1B7/CD3 BsAb to both TSLPR and CD3e antigens was assayed by Bio-Layer Interferometry. 1B7 alone served as the positive control for TSLPR binding, and negative control for CD3e binding. A BSA loaded sensor with TSLPR and CD3e served as the negative control for both antigen binding.