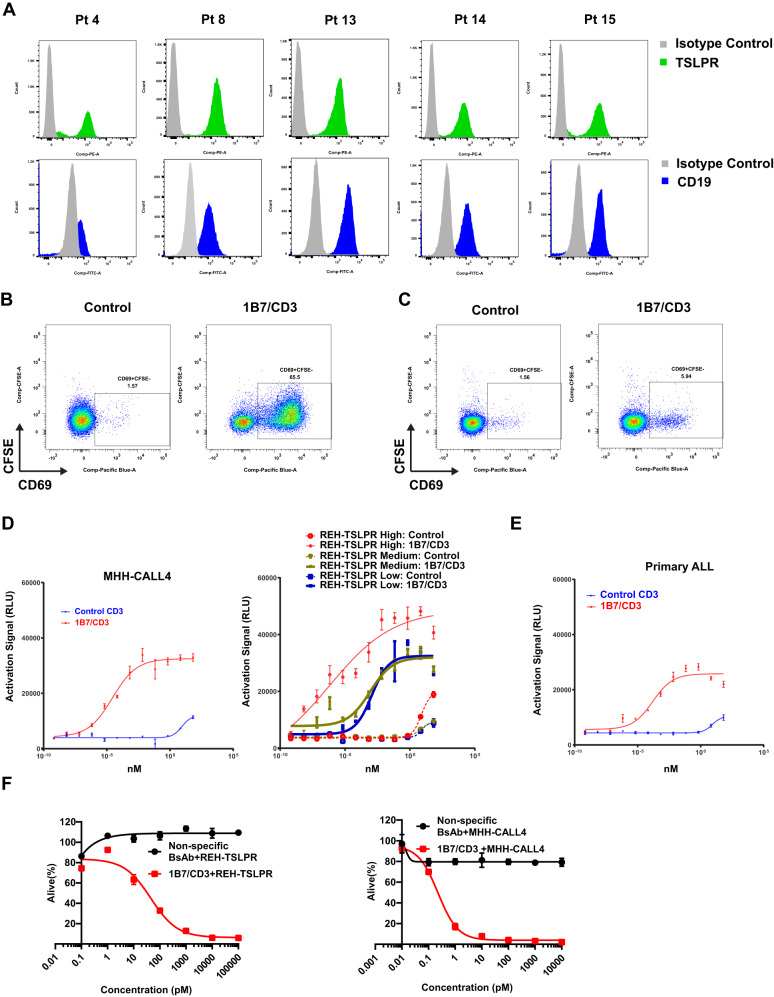
Fig. 2. 1B7/CD3 bispecific antibody (BsAb) induced a strong antigen-specific T cell activation and tumor cell killing in vitro.
A Comparable expression of TSLPR and CD19 in primary B-cell acute lymphocytic leukemia (B-ALL) samples. TSLPR and CD19 expression were determined on gated live CD45/TSLPR or CD45/CD19 double-positive cells respectively. B Activation of CD8 + T cells by 1B7/CD3 BsAb. REH-TSLPR cells (GFP+) were incubated with 1B7/CD3 BsAb or a control (inactive) BsAb before addition of human primary CD8 + T cells followed by culturing for 48 h. Levels of the activation marker CD69 are shown on CD3 + CD8 + GFP− cells. C 1B7/CD3 BsAb shows low levels of activation with human PBMC. 1B7/CD3 or inactive control BsAb 10 µg/ml were added to peripheral blood mononuclear cells (PBMC) from a normal donor. Levels of CD69 expression, as an indication of CD8 + T cell activation, were measured. D, E Expression of the NFAT luciferase reporter was triggered by 1B7/CD3 BsAb, but not the control antibody, in a reporter assay. D Low, medium, and high TSLPR expression (~1000, 2500, and 30,000 receptors/cell, respectively) in REH cells. Expression of TSLPR in (D) MHH-CALL4 ALL cells (native expressers of TSLPR, ~4000 copies) or in (E) cells from a primary patient sample (~6000 copies/cell). Cells were coated with 1B7/CD3 BsAb or control antibody before the addition of Jurkat cells expressing the NFAT luciferase reporter. The NFAT expression signal was measured 24 h. post activation and was expressed in relative light unit (RLU). F REH-TSLPR and MHH-CALL4 ALL cells were incubated with different concentrations of 1B7/CD3 BsAb or control antibody before adding human T cells at a 5:1 ratio (E: T). Flow cytometry was used to determine the cell viability after 48 h. treatment.