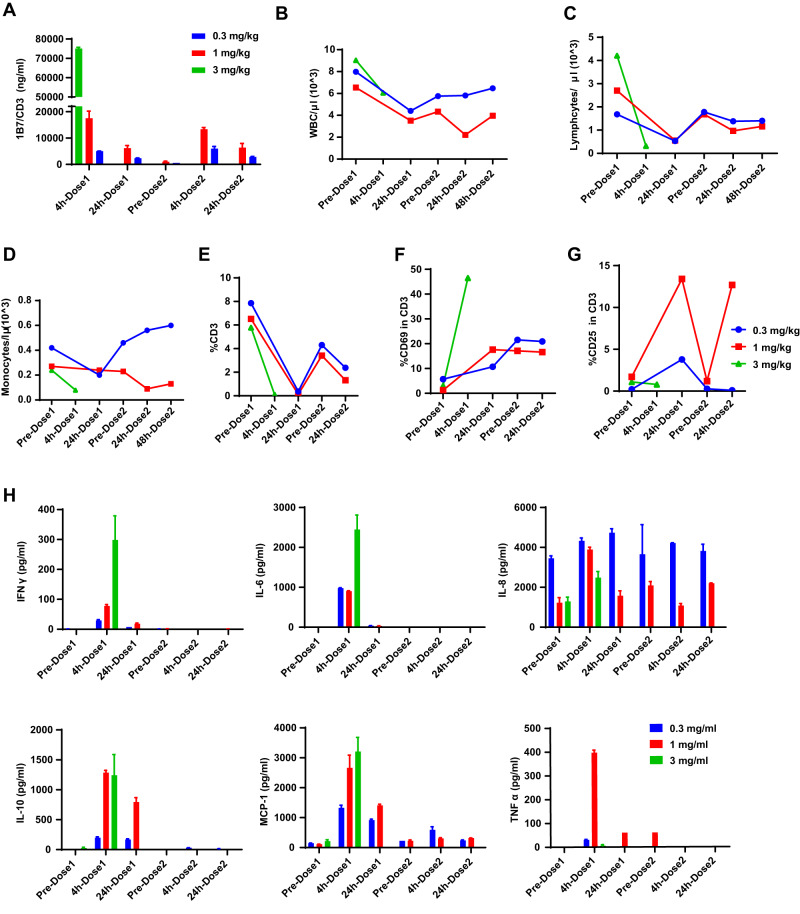
Fig. 6. Toxicity evaluation of the safety of 1B7/CD3 treatment in cynomolgus monkey.
Three adult, female cynomolgus monkeys were randomized into treatment groups and received 0.3, 1, or 3 mg/kg 1B7/CD3 via iv bolus on Day 1 and Day 8. Monkeys were observed at least twice daily for moribundity and mortality and were monitored closely for the first four hours following each dose administration. A 1B7/CD3 plasma exposure at indicated time points in cynomolgus monkeys treated with 0.3, 1, or 3 mg/kg 1B7/CD3. Data are shown as mean ± SD. B–D Concentrations of (B) white blood cells (WBC), (C) lymphocyte, and (D) monocyte at indicated time points in cynomolgus monkeys treated with 0.3, 1, or 3 mg/kg 1B7/CD3. Cell counts were performed by a hematological analyzer. E Dynamic change in CD3 positive T cells in the blood collected at indicated time points in cynomolgus monkeys treated with 0.3, 1, or 3 mg/kg 1B7/CD3. F, G Dynamic changes of (F) CD69 and (G) CD25 expression in T cells in the blood collected at indicated time points in cynomolgus monkeys treated with 0.3, 1, or 3 mg/kg 1B7/CD3. H Induction of cytokine levels by 0.3, 1, or 3 mg/kg 1B7/CD3 treatment in cynomolgus monkeys at indicated time points.