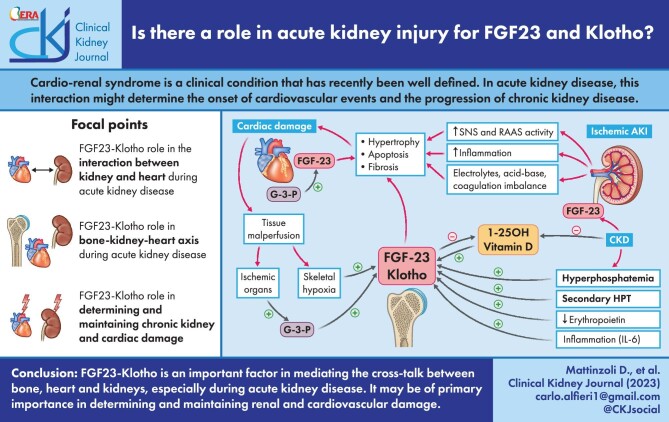
ABSTRACT
Cardio-renal syndrome is a clinical condition that has recently been well defined. In acute kidney disease, this interaction might trigger chronic processes determining the onset of cardiovascular events and the progression of chronic kidney disease. Moreover, the high mortality rate of acute kidney injury (AKI) is also linked to the fact that this condition is often complicated by dysfunctions of other organs such as lungs or heart, or is associated with septic episodes.
In this context the role and the potential link between bone, heart and kidney is becoming an important topic of research. The aim of this review is to describe the cardiac alterations in the presence of AKI (cardiorenal syndrome type 3) and explore how bone can interact with heart and kidney in determining and influencing the trend of AKI in the short and long term. The main anomalies of mineral metabolism in patients with AKI will be reported, with specific reference to the alterations of fibroblast growth factor 23 and Klotho as a link between the bone–kidney–heart axis.
Keywords: acute kidney injury, CKDMBD, FGF23, Klotho, mineral metabolism
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Cardio-renal syndrome is a clinical condition that has recently been well defined. From a generic point of view, cardiorenal syndrome can be defined as a clinical and pathological condition determined by anomalies affecting the heart and kidney, in which an acute or chronic functional alteration of one of the two organs significantly impacts the functionality of the other organ [1]. A close correlation in the presence of primary cardiac or renal damage emerges between heart and kidney both in chronic diseases and in acute conditions. In acute kidney injury (AKI), this interaction frequently might trigger chronic processes that determine the onset of cardiovascular events and the progression of chronic kidney disease (CKD) in the long term.
AKI, defined as an increase in creatinine of more than 1.5 times in 7 days or an increase in creatinine 0.3 mg/dL in the last 2 days associated with significant contraction of urine output [2], is a serious medical condition that is associated with a significant increase in mortality and length of hospital stay, and is associated with an important burden of healthcare costs. AKI complicates about 18% of hospital admissions—it is estimated that one in five adults experience an AKI episode during a hospital stay and it is associated with an in-hospital mortality of about 11% [3]. Furthermore, its incidence reaches up to 50% in intensive care, with a 27% mortality [4]. The high mortality rate of AKI is also linked to the fact that this condition is often complicated by dysfunctions of other organs such as lungs or heart, or is associated with septic episodes. Furthermore, AKI can represent a risk factor for progression to CKD [5]. In particular, the first 90 days after resolution of the acute event seem to be of paramount importance in identifying those patients at greater risk of developing chronic renal failure in the long term.
In this context the role and the potential link between bone, heart and kidney is becoming an important topic of research. Fibroblast growth factor 23 (FGF23) is a phosphatonin with endocrine capacity derived from bone, in particular from osteocytes. The classic action of FGF23 provides for the simultaneous presence of the co-receptor Klotho. In particular, in the presence of a CKD, FGF23 has been shown to have a phosphaturic capacity through the inhibition of phosphorus reabsorption at the level of the renal proximal tubule and vitamin D regulation [6]. Some recent evidence has also demonstrated independent Klotho effects of FGF23, some of which will be better presented in this article [7].
Klotho is a membrane protein that classically has a co-receptor function for FGF23. Recent research has demonstrated a systemic efficacy, also FGF23 independent of the cleaved and circulating form of Klotho [8].
The aim of the present review is to describe the interaction between kidney, heart and bone in the presence of AKI. In particular, after the first part in which some knowledge concerning the strong interaction between kidney and heart will be presented, the main anomalies of mineral metabolism in patients with AKI will be reported, with specific reference to the role of FGF23 and Klotho as a link in the bone–kidney–heart axis.
THE INTERACTION BETWEEN KIDNEY AND HEART IN THE PRESENCE OF AKI
Kidney–heart interaction is common in patients with AKI and a primary disorder of one of these two organs can suddenly result in secondary anomaly of the other. It can be viewed as a vicious circle: the de-compensation of the secondary organ might increase that of the first organ.
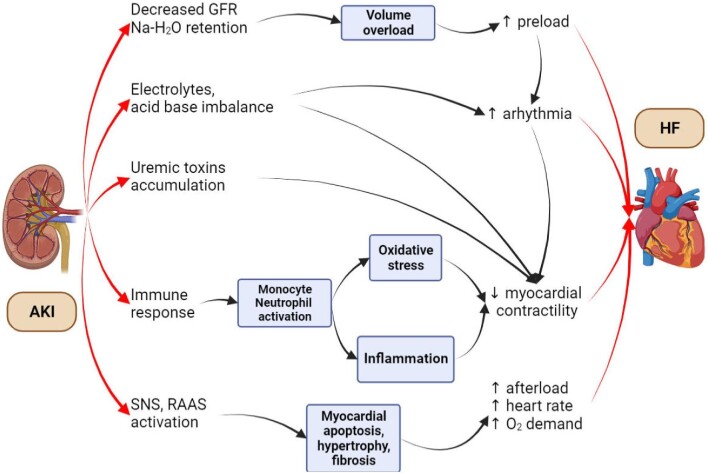
In a paper published in 1998 by Liaño et al. it was clearly demonstrated that, in patients with AKI admitted to intensive care units, hypotension was present in 60% of cases and was associated with an 81% mortality [9]. The influence of AKI on heart dynamism depends on various factors, ranging from an increase in preload (sodium and water retention and the increase of the volume overload ) and afterload [activation of the sympathetic nervous and renin–angiotensin–aldosterone systems (RAAS)], to an increase in the risk of arrhythmia (electrolytes and acid–base unbalance, accumulation of organic toxins, etc.). In addition, the immune activation typical of AKI might contribute to the development of chronic myocardial damage (Fig. 1).
Figure 1:
Interconnection between AKI and heart function impairment. AKI triggers cardiac dysfunction through various pathophysiological mechanisms, including volume overload, electrolyte and acid–base imbalances, accumulation of uremic toxins, enhanced immune response and activation of sympathetic nervous system and RAAS. HF: heart failure, GFR: glomerular filtration rate; SNS: sympathetic nervous system.
In the study published by Wu et al. in 2014, 4869 patients who recovered from dialysis-requiring AKI (AKI recovery group) were matched with 4869 patients without AKI (non-AKI group). Of note, patients in the AKI recovery group were characterized by a higher long-term risk for coronary events and all-cause mortality regardless of subsequent progression to CKD. This demonstrates the independent association between AKI and long-term cardiovascular risk [10].
More recent data, reported in a meta-analysis published in 2017, have clearly demonstrated that AKI is strictly related to cardiovascular events and represents a risk factor for the development of cardiovascular mortality, congestive heart failure, acute myocardial infarction and stroke [11].
The mechanism by which AKI can determine the onset of acute and then chronic heart damage over the years is correlated to the fact that days after the onset of AKI, processes of cellular necrosis, apoptosis and intracellular calcium mishandling take place at the myocardial level. These processes result in cardiac hypertrophy and fibrosis over the long term. Systemic inflammation, immune infiltration, and the activation of RAAS and the sympathetic nervous system are strongly implicated. In addition, oxidative stress and endothelial dysfunction which are typical of acute renal failure play an important role [12].
Of particularly high interest is also the mechanism in which, in presence of AKI, heart disease might increase the kidney damage. All the anomalies described above are responsible for the alteration of the volume status in AKI patients. Specifically, venous congestion by means of the elevation of renal venous pressure might be a significant contribution to the kidney insult, maintaining the kidney–heart vicious circle [13, 14].
HOW MIGHT THE BONE BE INCLUDED IN KIDNEY–HEART AXIS IN THE PRESENCE OF AKI? THE ROLE OF FGF23 AND KLOTHO
Compared with the classic anomalies of mineral and bone metabolism in CKD, much less is currently known about the effects of mineral metabolism dysregulation and inflammation on bone health during AKI. The main alterations that occur at the level of mineral metabolism in relation to chronic kidney dysfunction, such as hyperparathyroidism, hypocalcemia, hyperphosphatemia and hypovitaminosis D, might also develop during AKI [15]. In recent years a lot of interest has developed in the role of FGF23 and Klotho in AKI patients.
The first evidence for an elevation of FGF23 during AKI came from a clinical case presented in 2010 by Leaf et al. In this report, FGF23 reached very high levels (up to 619 RU/mL; normal value <100 RU/mL) at Day 7 post-admission in a patient with AKI induced by rhabdomyolysis [16].
A few years later, the same research group showed that FGF23 increased and was associated with severe AKI in patients who underwent cardiac surgery. The interesting finding is that the increase in FGF23 was mainly related to the increase in its C-terminal portion, with a significant increase in the cFGF23/iFGF23 ratio [17].
It must be said that even if the elevated levels of FGF23 are also found in the presence of CKD, the characteristics of FGF23 elevation in the presence of AKI are probably different and with different causes. First of all, what has been clearly demonstrated through the evaluation of FGF23 at renal vein artery level in the presence of AKI in patients undergoing to the catheterization of the heart is that, during AKI, there is probably an impaired excretion of FGF23 [18]. Moreover, in the setting of AKI, an increase in the expression of FGF23 was noted in the thymus, spleen and heart of mice treated with folic acid [19].
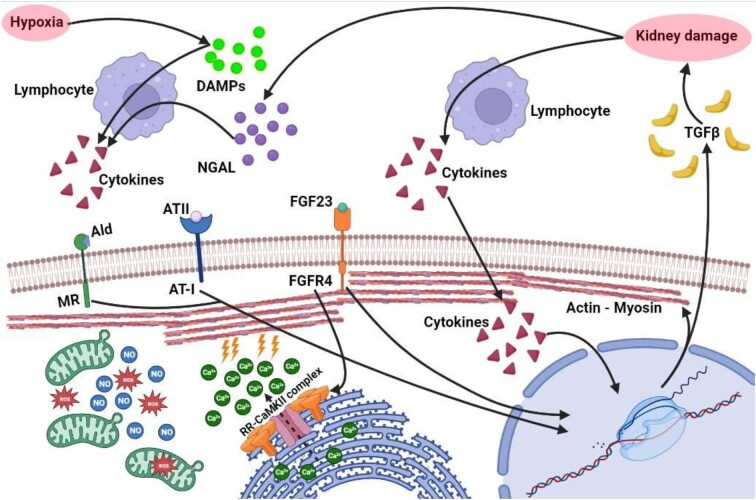
Several recently published articles have demonstrated the consequence of AKI in the predisposition of cardiomyocytes to trigger arrhythmias and a heart failure phenotype, and in relationship with high elevation in FGF23 and the decrease in Klotho levels. In a recent paper by González-Lafuente et al., the crosslink between mineral diseases and cardiac alteration in AKI was explored. In particular, in an experimental folic acid injection AKI model, high levels of FGF23, heart hypertrophy and an increase in systolic Ca2+ were rapidly demonstrated. Then, pro-arrhythmogenic Ca2+ events and ventricular arrhythmias were demonstrated in relation to the activation of the calcium/calmodulin-dependent kinase II pathway. Moreover, in the pilot study performed in 29 AKI patients the combination between FGF23 and phosphorus with troponin T levels resulted in a better prediction of the hospital mortality risk. The result of this study opens the discussion about the possible role of “biomarker of risk” for FGF23 in the AKI setting [20]. In Fig. 2 the effect of acute kidney damage combined with the elevation of circulating FGF23 on cardiac muscle cells is summarized.
Figure 2:
Effect on cardiomyocytes of kidney damage increased FGF23 levels and hypoxia. Kidney damage induces the production of NGAL and G-3-P, while an hypoxic milieu stimulates the production of DAMPs. Together these molecules induce the production of several pro-inflammatory cytokines by immune cells (IL-1, IL-6, TNF-α). This creates a pro-inflammatory environment which increases the production of NO and ROS and activates several transcription factor in cardiomyocytes, which lead to further production of pro-inflammatory mediators and TGF-β, which promotes further fibrosis and kidney damage. Moreover, in the context of renal hypoperfusion, as previously seen, there is an increase in SNS and RAAS activity. In addition, there is an increase in FGF23 levels, which binds directly on specific receptor on heart cells. These mechanisms act synergistically in augmenting intracellular Ca2+ levels by binding to RR and activating RR–CaMKII complex (especially FGF23), leading to calcium efflux from sarcoplasmic reticulum to cytoplasm. The increase in cytoplasmic calcium can lead to conduction abnormalities and predisposition to cardiac arrhythmias. FGF23 also contributes in increasing the synthesis of sarcomeric proteins, such as actin and myosin, thus inducing cardiac hypertrophy. DAMPs: damage-associated molecular pattern; G-3-P: galectin-3-phosphate; NGAL: neutrophil gelatinase-associated lipocalin; TGF-β: transforming growth factor β; Ald: aldosteron; MR: mineralcorticoid receptor; ATII: angiotensin II; AT-1: receptor for angiotensin II; NO: nitric oxide; ROS: reactive oxygen species; RR: ryanodine receptors.
Furthermore, experimental models of AKI induced by folic acid have shown that the expression of Klotho in the renal tubules is significantly reduced, and no production of the soluble form of Klotho is present [21, 22].
WHAT CAN BE THE POTENTIAL ROLE OF FGF23 AND KLOTHO IN AKI?
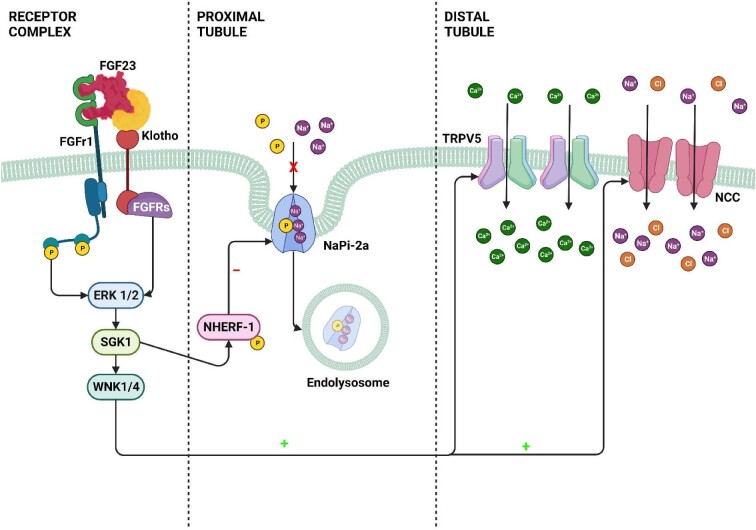
FGF23 is predominantly produced at the osteocyte level and targets the proximal tubule [23]. Its classical effect, mediated by the activation of its receptor, in cooperation with Klotho, is to mediate the internalization of the sodium phosphate cotransporter, thus allowing an increase in the excretion of phosphate in the urine. In a work published in 2014, it was shown that the administration of recombinant FGF23 resulted in a significant increase in the expression of the activity of Na–Cl co-transporters. This process was clearly demonstrated with western blot and immunofluorescence experiments. The increased activity of the Na–Cl co-transporters results in the increase of serum sodium and in the reduction of urinary volume and urinary sodium. Interestingly, treated mice developed severe left ventricular hypertrophy. The selective inhibition of the activity of these transporters, obtained through the administration of chlorothiazide, totally blocked the effect of FGF23. Figure 3 shows the main mechanism by which FGF23 influences tubular resorptive capacity. Considering these results, it can be assumed that FGF23, also in AKI states, could represent a determining factor in the development of the hypervolemia typical of these patients [24]. A recent study performed in the general population involving more than 6000 subjects has identified FGF23 as a promising biomarker to identify individuals at risk for the development of heart failure with reduced ejection fraction independently of potential confounders [25].
Figure 3:
FGF23–Klotho effects on tubular absorption mechanisms. FGF23–Klotho signaling in the kidney. In proximal renal tubules, blood-borne FGF23 binds to a receptor complex consisting of FGFRs and αKlotho (Klotho), and activates a signaling cascade involving ERK1/2 and SGK1. SGK1 in turn phosphorylates NHERF-1, leading to internalization and degradation of NaPi-2a. FGF23-induced phosphorylation of NHERF-1 decreases the membrane abundance of NaPi-2a, and leads to increased urinary phosphate excretion. The FGF23 signaling–induced mechanisms downstream of ERK1/2 which suppress the transcription of 1α-hydroxylase in proximal renal tubules are unknown. In distal renal tubules, FGF23 circulating in blood binds to the FGFR–Klotho receptor complex, and activates ERK1/2, SGK1 and the WNK1/4 complex. Activation of WNK signaling increases the luminal membrane abundance of glycosylated TRPV5 and of NCC, leading to increased distal tubular calcium and sodium reabsorption. ERK 1/2: Extracellular signal-regulated kinases 1/2; SGK1: Serum and glucocorticoid‐inducible kinase 1; WNK 1/4: Lysine Deficient Protein Kinase 1/4; NHERF-1: sodium–hydrogen exchanger regulatory factor; TRPV5: Transient Receptor Potential Vanilloid 5; NCC: Na+–Cl– cotransporter.
Furthermore, what has been reported in patients with AKI is that FGF23 and aldosterone could have a synergistic effect in the development of hypertension, often present in these patients. Experimental models have shown that following the exposure to corticosterone acetate or following salt depletion, the mouse models have a significant increase in the serum concentration of cFGF23, and in the transcription of FGF23 at the bone level [26].
FGF23 appears to act and trigger the activation of the local RAAS, promoting cardiac hypertrophy and fibrosis. In 5/6 nephrectomized rats—and therefore in a model of CKD and not AKI—after stimulation with FGF23, there is a significant increase in the expression of genes related to the RAAS [27]. Furthermore, if newborn rat ventricular myocytes are isolated and treated with FGF23 for 90 min, they develop a significant increase in the expression of angiotensin II, similar to that observed after the exposure to high concentrations of glucose [28]. It could be that in AKI settings these pathway may however be involved, as supposed in the ischemia–reperfusion model [29].
In a recently published work, aimed at identifying potential kidney-derived mediators of FGF23 production, Simic et al. identified a very close correlation between FGF23 and glycerol-3-phosphate, an acute marker of ischemic damage. Interestingly, wild-type mice treated with glycerol-3-phosphate developed an increase in the expression of FGF23 in bone and in bone marrow. Furthermore, in a mouse model of ischemic–reperfusion nephropathy and in subjects undergoing cardiac surgery, glycerol-3-phosphate was found to be an even earlier marker than creatinine in identifying the AKI event. This is a classic example of cross-talk between kidney and bone: glycerol-3-phosphate which is produced by the damaged kidney, further moves to the bone, increasing the production of FGF23 [30].
The role of FGF23 in the context of inflammation during AKI has also been examined by recent studies. It is known that in a model of folic acid–induced nephropathy, as well as in patients with AKI, the levels of numerous cytokines, including interleukin (IL)-10, IL-6 and tumor necrosis factor (TNF)-α increase in serum. This proinflammatory environment has an important impact on the transcription of the hypoxia-inducible factor 1 (HIF1) complex, a factor that has now been clearly demonstrated to be involved in the regulation of FGF23 cleavage and therefore in the consequent increased production of cFGF23 [31]. This has been brilliantly demonstrated in other studies which have clearly indicated that in AKI FGF23 levels are significantly increased mainly due to its C-terminal form. Consequently, it is correct to assume that the state of inflammation, typical of AKI, can be a great stimulus to increase the production of FGF23 [32]. On the other hand, it must be remembered that FGF23 would seem to be significantly involved in promoting and maintaining the inflammatory milieu typical of acute renal failure. In vivo studies have shown that, in adult wild-type mice treated with recombinant FGF23 administered intravenously, there is a significant increase in hepatic expression of C-reactive protein (CRP) and IL-6, and this is also associated with a contextual increase in serum CRP [33]. It is very interesting to note how FGF23 is able to increase serum levels of inflammatory cytokines independently from Klotho, involving the FGFR4 receptor. This could open future treatment options.
In our renal research laboratory, the detrimental relationship between FGF23 and inflammation in cardiovascular disease (CVD) has been well explored. First, based on the data reported by Singh's group, we found that the addition of FGF23 caused a biphasic effect on the hepatic production of Fetuin-A (acute phase glycoprotein with recognized anti-calcification activity) in a TNF-α-dependent manner [33, 34]. Subsequently, our group described and elucidated the post-transcriptional counterregulatory effect on this cardiovascular protector performed by the inflammatory mechanism TNF-α/nuclear factor (NF)-κB stimulated by FGF23 [35]. Second, we also explored the close association between FGF23, inflammation and increased risk of CVD from another perspective, noting that during the progression of kidney disease, the increased interplay between monocyte chemoattractant protein-1 (MCP1) and FGF23 creates an imbalance of the serum fatty acid profile in favor of Omega-6 fatty acids, precursors of the proatherogenic eicosanoid [36].
In the future, all these findings could be the object of research in AKI settings [34].
Particularly important in the contest of AKI-associated oxidative stress is the role of Klotho. Several years ago, a reduced expression of Klotho was noted in renal tubular cells exposed to H2O2 [37]. This reduction in Klotho expression was time- and dose-dependent. More recently, a protective role has been shown for Klotho against myocardial hypertrophy induced by inositol sulfate [38]. What appears to be the basis of this process is a Klotho-dependent mechanism of oxidative stress regulation, which could therefore significantly impact systemic damage in the presence of AKI. The relationship between Klotho and oxidative stress regulation in kidney diseases has been reviewed recently by Donate-Correa et al. [39]. In AKI, Klotho might have serious implications in the protection from mitochondrial disfunction [40]. The reductions of Klotho levels are in fact highly correlated with the degree of mitochondrial disfunction [41].
As already explained, inflammation plays a fundamental role in the onset and maintenance of AKI. Experimental data have shown that pro-inflammatory factors, such as NF-κB, TNF-like weak inducer of apoptosis (TWEAK) and TNF-α, can determine the reduction of Klotho levels typical of patients with AKI [21]. Furthermore, Klotho silencing in a rhabdomyolysis model of acute renal failure is associated with an increased expression of the inflammatory factors TNF-α and IL-1β [42]. In this case, as in others, there would therefore seem to be a real vicious circle that is created between inflammation and Klotho levels.
In accordance to this hypothesis, a recent paper published by Junho et al., the therapeutic role of Klotho in cardiorenal syndrome (ischemia–reperfusion model) has been investigated. After Klotho treatment for 8 days determined a prevention in the increase of IL-6, IL-1β and TNF-α with a positive effect also in the modulation of the cardiac Ca2+ release, resulting in a prevention of arrhythmic events [43].
The same group has recently investigated the importance of the action of Klotho in the heart in AKI, by means of a AKI overdose of folic acid model comparing wild-type and heterozygous hypomorphic mice for the Klotho gene (+/kl). Interestingly, the heart contraction was decreased in +/kl mice. Those mice also showed a dysregulation in Ca2+ transients in systole and an disarrangement of sarco/endoplasmic reticulum Ca2+-ATPase, resulting in pro-arrhythmic events [44]. These recent findings might validate the effect of the decreased levels of Klotho in inflammation, oxidative stress, mitochondria abnormalities and finally cellular senescence, and its potential therapeutic utility in AKI setting [45]. The use of Klotho as therapeutic agent in AKI has been explored in some studies. In a model of ischemia–reperfusion AKI, Klotho gene induction was followed by an improvement of serum creatinine and the histological changes, and an attenuation of the apoptosis induced in the model [46]. More recently, recombinant αKlotho administration after AKI accelerated renal recovery and reduced renal fibrosis in bilateral ischemia–reperfusion injury and unilateral nephrectomy plus contralateral ischemia–reperfusion injury [47].
Endothelial dysfunction is a crucial point in these patients, in which both inflammation and oxidative stress play a fundamental role in determining a reduction in endothelial nitric oxide synthase (eNOS) activity with the consequent development of endothelial dysfunction and increased cardiovascular risk in both the short and long term. Therefore, reactive oxygen species metabolism and endothelial proliferation can be induced due to the dysregulation of the FGF23 axis, leading to the production of free radicals and vascular damage typical of patients with AKI [48].
CONCLUSIONS
In conclusion, it is now evident that especially in the presence of AKI, the cross-talk between bone, heart and kidneys may be of primary importance in determining and maintaining not only renal damage but also acute and chronic cardiovascular damage. Pharmacological interventions on the FGF23–Klotho axis could be important in the future in the management of AKI. Furthermore, a better understanding of the link between kidney, heart and bone in AKI could allow a better management and monitorization of the acute event therefore reducing the future risk of CKD. In this context, a potential role for FGF23 and Klotho as disease biomarkers might be explored [49].
ACKNOWLEDGEMENTS
The authors thank Marina Balderacchi for the kind collaboration in the health research and in the realization of this study.
Contributor Information
Deborah Mattinzoli, Department of Nephrology, Dialysis and Renal Transplantation, Fondazione IRCCS Ca’ Granda Ospedale Policlinico, Milan, Italy.
Paolo Molinari, Department of Nephrology, Dialysis and Renal Transplantation, Fondazione IRCCS Ca’ Granda Ospedale Policlinico, Milan, Italy; Post-Graduate School of Specialization in Nephrology, University of Milan, Milan, Italy.
Gregorio Romero-González, Department of Nephrology, Germans Trias i Pujol University Hospital, Research Group on Renal Diseases (REMAR), Germans Trias i Pujol Research Institute, Badalona, Spain.
Jordi Bover, Department of Nephrology, Germans Trias i Pujol University Hospital, Research Group on Renal Diseases (REMAR), Germans Trias i Pujol Research Institute, Badalona, Spain.
Elisa Cicero, Department of Nephrology, Dialysis and Renal Transplantation, Fondazione IRCCS Ca’ Granda Ospedale Policlinico, Milan, Italy; Post-Graduate School of Specialization in Nephrology, University of Milan, Milan, Italy.
Francesco Pesce, Nephrology, Dialysis and Transplantation Unit Department of Precision and Regenerative Medicine and Ionian Area (DiMePre-J) University of Bari “Aldo Moro, ”.
Matteo Abinti, Department of Nephrology, Dialysis and Renal Transplantation, Fondazione IRCCS Ca’ Granda Ospedale Policlinico, Milan, Italy; Post-Graduate School of Specialization in Nephrology, University of Milan, Milan, Italy.
Costanza Conti, Department of Nephrology, Dialysis and Renal Transplantation, Fondazione IRCCS Ca’ Granda Ospedale Policlinico, Milan, Italy; Post-Graduate School of Specialization in Nephrology, University of Milan, Milan, Italy.
Giuseppe Castellano, Department of Nephrology, Dialysis and Renal Transplantation, Fondazione IRCCS Ca’ Granda Ospedale Policlinico, Milan, Italy; Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy.
Carlo Alfieri, Department of Nephrology, Dialysis and Renal Transplantation, Fondazione IRCCS Ca’ Granda Ospedale Policlinico, Milan, Italy; Department of Clinical Sciences and Community Health, University of Milan, Milan, Italy.
DATA AVAILABILITY STATEMENT
No new data were generated or analyzed in support of this research.
FUNDING
Publication costs were funded by Grant Ricerca Corrente, Italian Ministry of Health.
CONFLICT OF INTEREST STATEMENT
J.B. is member of the CKJ editorial board. The other authors declare no conflict of interest.
REFERENCES
- 1. Ronco C, Bellasi A, Di Lullo L. Cardiorenal syndrome: an overview. Adv Chronic Kidney Dis 2018;25:382–90. 10.1053/j.ackd.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 2. Kellum JA, Lameire N, Aspelin Pet al. . Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138. [Google Scholar]
- 3. Susantitaphong P, Cruz DN, Cerda Jet al. . Acute Kidney Injury Advisory Group of the American Society of Nephrology . World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013;8:1482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bedford M, Farmer C, Levin Aet al. . Acute kidney injury and CKD: chicken or egg? Am J Kidney Dis 2012;59:485–91. 10.1053/j.ajkd.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 5. Scaravilli V, Merrino A, Bichi Fet al. . Longitudinal assessment of renal function after lung transplantation for cystic fibrosis: transition from post-operative acute kidney injury to acute kidney disease and chronic kidney failure. J Nephrol 2022;35:1885–93. 10.1007/s40620-022-01392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodelo-Haad C, Santamaria R, Muñoz-Castañeda JRet al. . FGF23, biomarker or target? Toxins 2019;11:175. 10.3390/toxins11030175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grabner A, Mazzaferro S, Cianciolo Get al. . Fibroblast growth factor 23: mineral metabolism and beyond. Contrib Nephrol 2017;190:83–95. 10.1159/000468952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuro-O M. The Klotho proteins in health and disease. Nat Rev Nephrol 2019;15:27–44. 10.1038/s41581-018-0078-3. [DOI] [PubMed] [Google Scholar]
- 9. Liaño F, Junco E, Pascual Jet al. . The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. The Madrid acute renal failure study group. Kidney Int Suppl 1998;66:S16–24. [PubMed] [Google Scholar]
- 10. Wu VC, Wu CH, Huang TMet al. . Long-term risk of coronary events after AKI. J Am Soc Nephrol 2014;25(3):595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Odutayo A, Wong CX, Farkouh Met al. . AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol 2017;28:377–87. 10.1681/ASN.2016010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Legrand M, Rossignol P. Cardiovascular consequences of acute kidney injury. N Engl J Med 2020;382:2238–47. 10.1056/NEJMra1916393. [DOI] [PubMed] [Google Scholar]
- 13. Rubinstein J, Sanford D.. Treatment of cardiorenal syndrome. Cardiol Clin 2019;37:267–73. 10.1016/j.ccl.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 14. Hatamizadeh P, Fonarow GC, Budoff MJet al. . Cardiorenal syndrome: pathophysiology and potential targets for clinical management. Nat Rev Nephrol 2013;9:99–111. 10.1038/nrneph.2012.279. [DOI] [PubMed] [Google Scholar]
- 15. Leaf DE, Christov M.. Dysregulated mineral metabolism in AKI. Semin Nephrol 2019;39:41–56. 10.1016/j.semnephrol.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 16. Leaf DE, Wolf M, Stern L.. Elevated FGF-23 in a patient with rhabdomyolysis-induced acute kidney injury. Nephrol Dial Transplant 2010;25:1335–7. 10.1093/ndt/gfp682. [DOI] [PubMed] [Google Scholar]
- 17. Leaf DE, Christov M, Jüppner Het al. . Fibroblast growth factor 23 levels are elevated and associated with severe acute kidney injury and death following cardiac surgery. Kidney Int 2016;89:939–48. 10.1016/j.kint.2015.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Ballegooijen AJ, Rhee EP, Elmariah Set al. . Renal clearance of mineral metabolism biomarkers. J Am Soc Nephrol 2016;27:392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egli-Spichtig D, Zhang MYH, Perwad F.. Fibroblast growth factor 23 expression is increased in multiple organs in mice with folic acid-induced acute kidney injury. Front Physiol 2018;9:1494. 10.3389/fphys.2018.01494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. González-Lafuente L, Navarro-García JA, Rodríguez-Sánchez Eet al. . Interplay between mineral bone disorder and cardiac damage in acute kidney injury: from Ca2+ mishandling and preventive role of Klotho in mice to its potential mortality prediction in human. Transl Res 2022;243:60–77. 10.1016/j.trsl.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 21. Moreno JA, Izquierdo MC, Sanchez-Niño MDet al. . The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol 2011;22:1315–25. 10.1681/ASN.2010101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mencke R, Harms G, Moser Jet al. . Human alternative Klotho mRNA is a nonsense-mediated mRNA decay target inefficiently spliced in renal disease. JCI Insight 2017;2:e94375. 10.1172/jci.insight.94375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andrukhova O, Zeitz U, Goetz Ret al. . FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone 2012;51:621–8. 10.1016/j.bone.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andrukhova O, Slavic S, Smorodchenko Aet al. . FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med 2014;6:744–59. 10.1002/emmm.201303716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Binnenmars SH, Hoogslag GE, Yeung SMHet al. . Fibroblast growth factor 23 and risk of new onset heart failure with preserved or reduced ejection fraction: the PREVEND study. J Am Heart Assoc 2022;11:e024952. 10.1161/JAHA.121.024952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang B, Umbach AT, Chen Het al. . Up-regulation of FGF23 release by aldosterone. Biochem Biophys Res Commun 2016;470:384–90. 10.1016/j.bbrc.2016.01.034. [DOI] [PubMed] [Google Scholar]
- 27. Böckmann I, Lischka J, Richter Bet al. . FGF23-mediated activation of local RAAS promotes cardiac hypertrophy and fibrosis. Int J Mol Sci 2019;20:4634. 10.3390/ijms20184634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mhatre KN, Wakula P, Klein Oet al. . Crosstalk between FGF23- and angiotensin II-mediated Ca2+ signaling in pathological cardiac hypertrophy. Cell Mol Life Sci 2018;75:4403–16. 10.1007/s00018-018-2885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deng LC, Alinejad T, Bellusci Set al. . Fibroblast growth factors in the management of acute kidney injury following ischemia-reperfusion. Front Pharmacol 2020;11:426. 10.3389/fphar.2020.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simic P, Kim W, Zhou Wet al. . Glycerol-3-phosphate is an FGF23 regulator derived from the injured kidney. J Clin Invest 2020;130:1513–26. 10.1172/JCI131190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. David V, Martin A, Isakova Tet al. . Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int 2016;89:135–46. 10.1038/ki.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Czaya B, Faul C.. FGF23 and inflammation-a vicious coalition in CKD. Kidney Int 2019;96:813–5. 10.1016/j.kint.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 33. Singh S, Grabner A, Yanucil Cet al. . Fibroblast growth factor 23 directly targets hepatocytes to promote inflammation in chronic kidney disease. Kidney Int 2016;90:985–96. 10.1016/j.kint.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mattinzoli D, Ikehata M, Tsugawa Ket al. . FGF23 and fetuin-a interaction in the liver and in the circulation. Int J Biol Sci 2018;14:586–98. 10.7150/ijbs.23256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mattinzoli D, Li M, Castellano Get al. . Fibroblast growth factor 23 level modulates the hepatocyte's alpha-2-HS-glycoprotein transcription through the inflammatory pathway TNFα/NFκB. Front Med (Lausanne) 2022;9:1038638. 10.3389/fmed.2022.1038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mattinzoli D, Turolo S, Alfieri CMet al. . MCP1 Could mediate FGF23 and omega 6/omega 3 correlation inversion in CKD. J Clin Med 2022;11:7099. 10.3390/jcm11237099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mitobe M, Yoshida T, Sugiura Het al. . Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp Nephrol 2005;101:e67–74. 10.1159/000086500. [DOI] [PubMed] [Google Scholar]
- 38. Yang K, Wang C, Nie Let al. . Klotho protects against indoxyl sulphate-induced myocardial hypertrophy. J Am Soc Nephrol 2015;26:2434–46. 10.1681/ASN.2014060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Donate-Correa J, Martín-Carro B, Cannata-Andía JBet al. . Oxidative stress, and mitochondrial damage in kidney disease. Antioxidants (Basel) 2023;12:239. 10.3390/antiox12020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Panesso MC, Shi M, Cho HJet al. . Klotho has dual protective effects on cisplatin-induced acute kidney injury. Kidney Int 2014;85:855–70. 10.1038/ki.2013.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sahu A, Mamiya H, Shinde SNet al. . Age-related declines in α-klotho drive progenitor cell mitochondrial dysfunction and impaired muscle regeneration. Nat Commun 2018;9:4859. 10.1038/s41467-018-07253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lin W, Wu X, Wen Jet al. . Nicotinamide retains Klotho expression and ameliorates rhabdomyolysis-induced acute kidney injury. Nutrition 2021;91-92:111376. 10.1016/j.nut.2021.111376. [DOI] [PubMed] [Google Scholar]
- 43. Junho CVC, González-Lafuente L, Neres-Santos RSet al. . Klotho relieves inflammation and exerts a cardioprotective effect during renal ischemia/reperfusion-induced cardiorenal syndrome. Biomed Pharmacother 2022;153:113515. 10.1016/j.biopha.2022.113515. [DOI] [PubMed] [Google Scholar]
- 44. González-Lafuente L, Navarro-García JA, Valero-Almazán Áet al. . Partial genetic deletion of Klotho aggravates cardiac calcium mishandling in acute kidney injury. Int J Mol Sci 2023;24:1322. 10.3390/ijms24021322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee J, Tsogbadrakh B, Yang Set al. . Klotho ameliorates diabetic nephropathy via LKB1-AMPK-PGC1α-mediated renal mitochondrial protection. Biochem Biophys Res Commun 2021;534:1040–6. 10.1016/j.bbrc.2020.10.040. [DOI] [PubMed] [Google Scholar]
- 46. Sugiura H, Yoshida T, Tsuchiya Ket al. . Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol Dial Transplant 2005;20:2636–45. 10.1093/ndt/gfi165. [DOI] [PubMed] [Google Scholar]
- 47. Shi M, Flores B, Gillings Net al. . αKlotho mitigates progression of AKI to CKD through activation of autophagy. J Am Soc Nephrol 2016;27:2331–45. 10.1681/ASN.2015060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Roumeliotis S, Mallamaci F, Zoccali C.. Endothelial dysfunction in chronic kidney disease, from biology to clinical outcomes: a 2020 update. J Clin Med 2020;9:2359. 10.3390/jcm9082359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roy C, Lejeune S, Slimani Aet al. . Fibroblast growth factor 23: a biomarker of fibrosis and prognosis in heart failure with preserved ejection fraction. ESC Heart Fail 2020;7:2494–507. 10.1002/ehf2.12816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated or analyzed in support of this research.