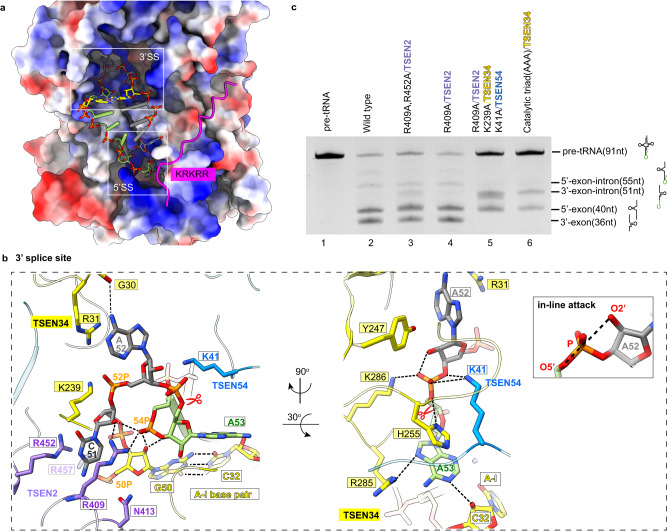
Fig. 4. Recognition and cleavage mechanisms of 3′ splicing boundary by human TSEN complex.
a Binding pockets for 3′ and 5′ splicing boundaries of tRNA molecules. Electrostatic surface of the body of the TSEN complex shows charged pockets. The A-I base pair is shown as yellow sticks. The nucleotides of the anticodon loop are in green, and those of the intron are in gray. The two splicing boundaries, shown as sticks, are located in deep pockets in TSEN. The N-terminal insertion of TSEN2, shown as cartoon in magenta, covers part of the 5′ splice center. The position of the positively charged motif is labeled. 3′SS: 3′ splice site; 5′SS: 5′ splice site. b Detail views of the 3′ splice site with TSEN34, TSEN2, and TSEN54. The 3′ bugle and A-I base pair are shown as sticks. C51 is cation-π interactions with TSEN2 R409 and R452. R409 forms hydrogen bonds with the backbones of both C51 and U54. TSEN2 R457 and N413 and TSEN34 R31 and K239 interact with the phosphate groups of G50 and A52, respectively. In the right most panel, the A53 base is surrounded by H255, R285, and the A-I base pair from three sides. The scissile phosphate, indicated with the symbol ‘scissors’, is located in the center of the catalytic triad of TSEN34 –Y247, H255, and K286, covered by TSEN54 K41 at the same time. The insert presents the in-line geometry created by the attacking 2′-nucleophile oxygen, scissile phosphate, and the leaving 5′-oxygen. c Cleavage assays showing that the R409A mutation alone had minimal impact (lane 4), whereas the double mutants R409A and R452A, resulting in the accumulation of the 3′-exon-intron intermediate, decreased the cleavage rate (lane 3). The triple mutants R409A (TSEN2), K239A (TSEN34), and K41A (TSEN54) completely abolished cleavage (lane 5), similar to mutating the catalytic triad of residues to Ala in TSEN34 (lane 6). The cleavage assays were repeated three times. Source data are provided as a Source data file.