Figure 5. PRMT1 interacts with WDR24 and methylates WDR24 at R329.
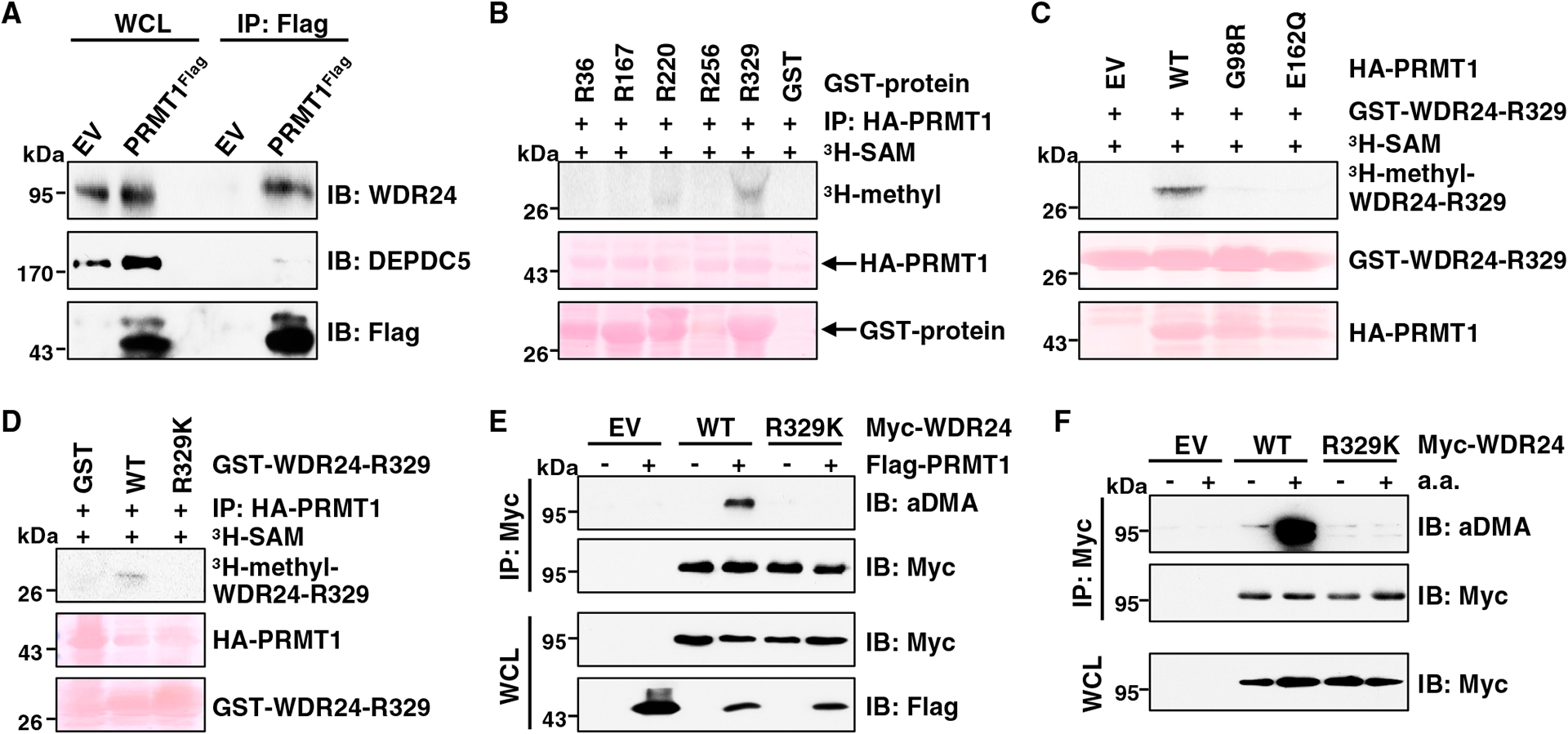
(A) IB analysis of WCLs and anti-FLAG IPs derived from HEK293T cells (EV, empty vector) and PRMT1FLAG knockin HEK293T cells. Similar results were obtained in three independent experiments.
(B) In vitro arginine methylation assays using recombinant GST-WDR24 truncated proteins purified from E. coli as substrates. HA-PRMT1 proteins immunopurified from HEK293T cells were used as the methyltransferase. Similar results were obtained in three independent experiments.
(C) In vitro arginine methylation assays using recombinant GST-WDR24-R329 protein as the substrate. HA-WT-PRMT1 or enzymatic-dead PRMT1 proteins derived from HEK293T cells were used as the methyltransferase. Similar results were obtained in three independent experiments.
(D) In vitro arginine methylation assays using recombinant GST-WDR24-R329-WT and GST-WDR24-R329K proteins as substrates. HA-WT-PRMT1 protein derived from HEK293T cells was used as the methyltransferase. Similar results were obtained in three independent experiments.
(E) IB analysis of WCLs and anti-Myc IPs derived from HEK293T cells transfected with indicated constructs. Similar results were obtained in three independent experiments.
(F) IB analysis of WCLs and anti-Myc IPs derived from HEK293T cells transfected with indicated constructs. Cells were starved of aa for 50 min or starved for 50 min and then restimulated with aa for 10 min before harvesting. Similar results were obtained in three independent experiments.
See also Figure S5.
