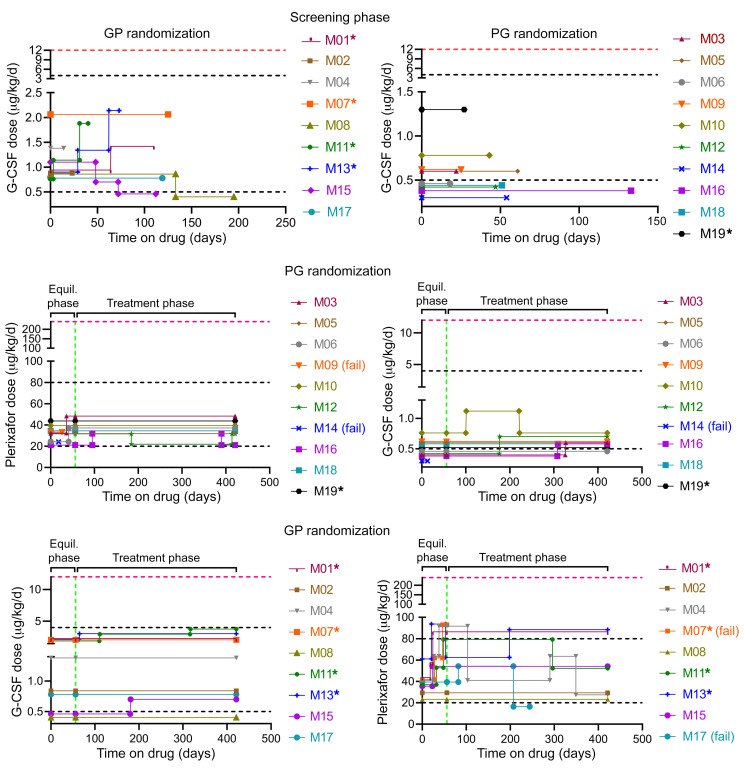
Figure 3. Low dose G-CSF versus plerixafor for 19 patients with WHIM.
Drug doses for the 3 phases of the study are shown, stratifying patients by randomization order. P, plerixafor; G, G-CSF. Horizontal dashed red lines indicate the package insert-recommended total daily dosage of G-CSF for severe congenital neutropenia or the single injection daily FDA-approved dose of plerixafor for HSC mobilization. Vertical dashed green lines indicate day 56, the final day of the equilibration phase (Equil. phase). Horizontal dashed black lines indicate target total daily dose ranges for the study. Children are indicated by asterisks. Changes in drug dose were to stay within the target ANC range or to mitigate side effects. (fail), patient dropouts due to side effects or drug failure (see main text for details).