Figure 5.
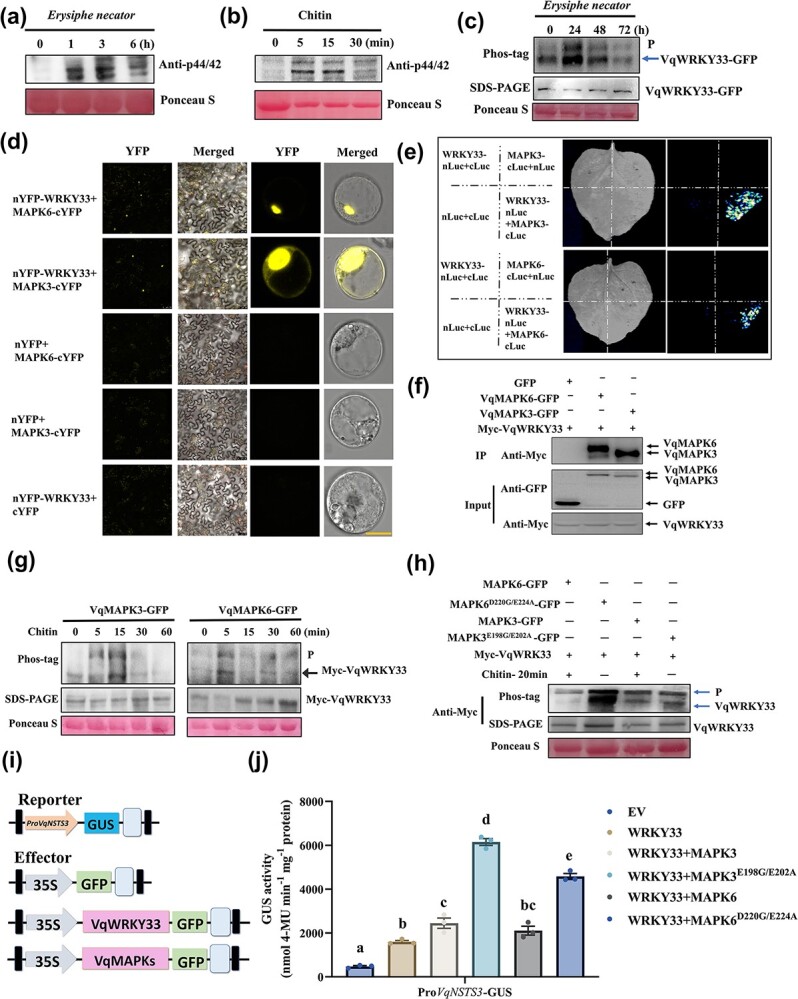
VqMAPK3/6 interact with and phosphorylate VqWRKY33, inducing the expression of VqNSTS3. a Activation of VqMAPKs in Danfeng-2 leaves treated with E. necator was verified by western blot assays. b Activation of VqMAPKs in Danfeng-2 leaves treated with 1 mg/ml chitin was verified by western blot assays. c Phosphorylation of VqWRKY33 was induced by E. necator. Proteins were separated by Phos-tag gel. d BiFC assays verified the interaction between VqWRKY33 and VqMAPK3/6 in tobacco leaves and grape protoplasts. Scale bars, 50/10 μm. e Split-luciferase complementation assays confirmed the interaction between VqWRKY33 and VqMAPK3/6. f CoIP assays validated that VqWRKY33 interacted with VqMAPK3 and VqMAPK6. g Phosphorylation of VqWRKY33 co-expressed with VqMAPK3 and VqMAPK6 after chitin treatment. Proteins were separated by Phos-tag gel, and then detected by immunoblotting with an anti-Myc antibody. h Phosphorylation of VqWRKY33 was induced by phospho-mimicking VqMAPK3/6 mutants. Proteins were separated by Phos-tag gel, and then detected by immunoblotting with an anti-Myc antibody. i Structural diagrams of GUS activity assays. j Measurement of GUS activity. ProVqNSTS3-GUS was co-transformed with 35S-GFP, 35S-VqWRKY33-GFP, and 35S-VqMAPKs-GFP in tobacco leaves. Results are shown as mean ± standard error of the mean; n = 3, and different letters represent significant differences (P < .05) as determined by one-way ANOVA followed by Tukey’s multiple comparisons test.
