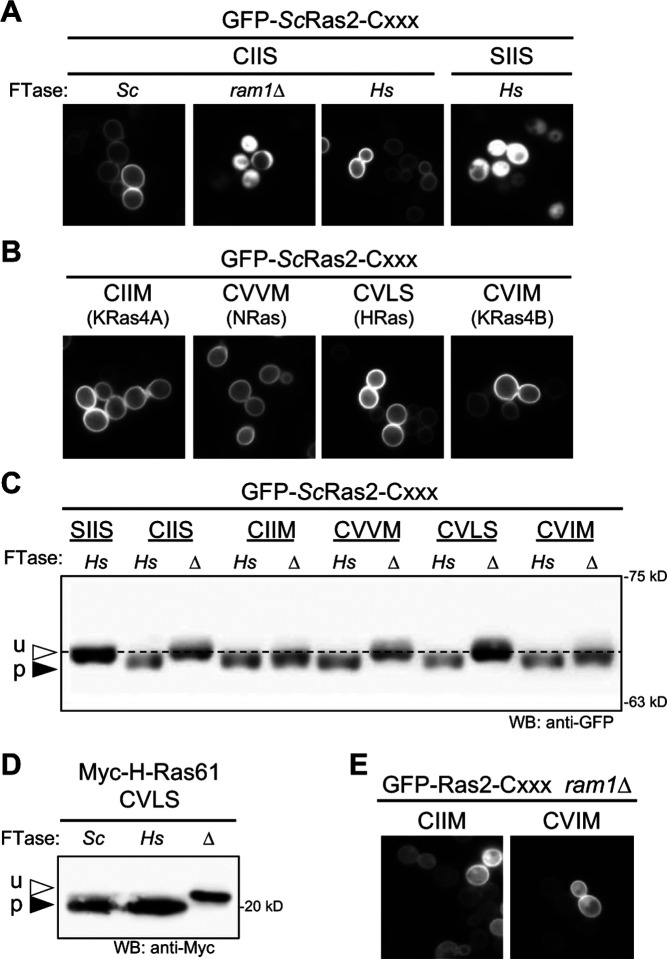
Figure 2. The humanized FTase strain fully modifies yeast and human Ras CaaX sequences.
GFP-Ras2 was used as a reporter to evaluate CaaX sequences derived from yeast Ras2 (CIIS) and human proteins KRas4A (CIIM), NRas (CVVM), HRas (CVLS), and KRas4B (CVIM). A) The localization of GFP-ScRas2 produced in wildtype (Sc), humanized (Hs), and FTase-deficient (ram1Δ) yeast strains was determined by fluorescence microscopy. GFP-ScRas2-SIIS is a mutant that cannot be prenylated and is cytosolically localized. B) The localization of GFP-ScRas2-CaaX variants encoding human Ras CaaX sequences produced in the humanized FTase strain was determined by fluorescence microscopy. The source of the CaaX sequence is indicated below each specific sequence. C) Western blot analysis of GFP-ScRas2-CaaX variants produced in humanized (Hs) or FTase-deficient (Δ) yeast strains. Total cell lysates were prepared, and equivalent protein amounts analyzed by SDS-PAGE and Western blot using anti-GFP antibody. u –unprenylated GFP-ScRas2; p – prenylated GFP-ScRas2. The dashed line was aligned with unprenylated GFP-Ras2 to serve as a visual reference. The plasmids used in panels A-C were pWS1735 (GFP-ScRas2), pWS1889 (GFP-ScRas2-SIIS), pWS1997 (GFP-ScRas2-CIIM), pWS1998 (GFP-ScRas2-CVVM), pWS1999 (GFP-ScRas2-CVLS), and pWS2000 (GFP-ScRas2-CVIM). pWS1889 encodes a double cysteine to serine mutation; the upstream cysteine is typically palmitoylated but was mutated to avoid creation of a non-canonical length CaaaX sequence. Yeast strains used were BY4741 (wildtype ScFTase), yWS3220 (HsFTase), and yWS3202 (ram1Δ). D) Myc-HRas61 (p-05547) was produced in wildtype (Sc; yWS2544), humanized FTase (Hs; yWS3186), or FTase-deficient (Δ; yWS3209) yeast strains. HRas61 is the Q61L oncogenic derivative of human HRas (Adari et al., 1988; Farnsworth et al., 1991). E) The localization of GFP-ScRas2-CaaX variants (CIIM and CVIM) that undergo alternate prenylation by ScGGTase-I in the absence of FTase (i.e., ram1Δ strain background).