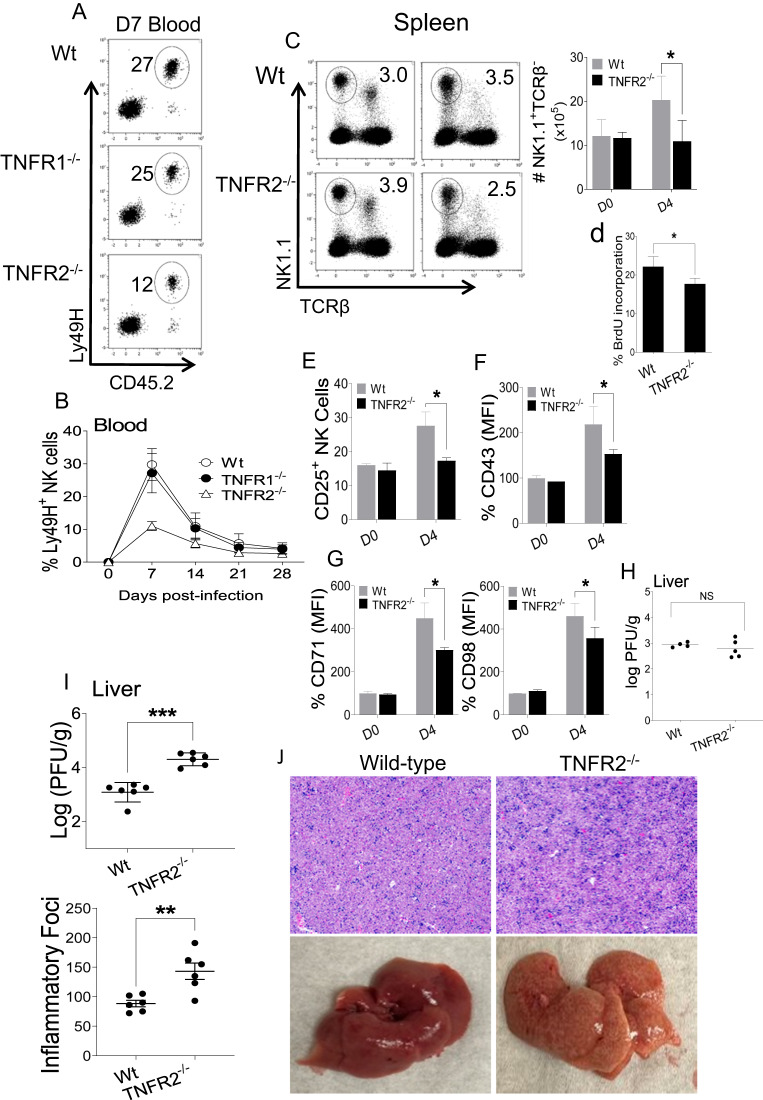
Fig. 8.
Intrinsic signaling through TNFR2 for NK cell proliferation during acute MCMV infection in vivo. Splenic NK cells of C57BL/6, TNFR1−/−, and TNFR2−/− mice (CD45.2+) were isolated and transferred into Ly49H−/− recipient (CD45.1+) mice. Ly49H−/− mice were either uninfected or intraperitoneally given 3000 PFU MCMV one day following adoptive transfer. Peripheral leukocytes in the blood of recipient mice were harvested and analyzed every 7th day post-infection. A Representative plots show transferred cells (Ly49H+CD45.2+) gated on DX5+TCRβ- cells in the blood of recipient mice on Day 7. B Representative graph of transferred cells (Ly49H+CD45.2+) gated on DX5+TCRβ- cells from the blood of recipient mice on the indicated days post-infection. C57BL/6 and TNFR2−/− mice were either uninfected or intraperitoneally challenged with 3000 PFU MCMV, and splenic leukocytes were analyzed from naive (D0) or infected mice on Day 4 (D4) post-infection to assess (C) NK cell proportion and (D) BrdU incorporation of NK cells from the spleens of the indicated mice. Representative graphs represent the (E) proportion of CD25+ cells among the total NK cell population, (F) MFI of CD43, (G) CD71 and CD98 expression in NK cells from indicated mice, and (H) viral titers in the liver of indicated mice. Equal numbers (50,000) of purified splenic Ly49H+ NK cells from wild-type and TNFR2−/− mice were transferred into Rag2−/−Il2rg−/− mice. Mice were intraperitoneally infected with MCMV one day following adoptive transfer. On Day 7 post-infection, livers were collected to assess (I) viral titer and (J) inflammatory foci through H&E staining. Data are from one experiment representative of two independent experiments, with at least 4 mice per group. The MFI expression is presented as a percentage relative to the MFI of control mice as 100. Data represent the mean ± SD. *p < 0.05; **p < 0.01; ***p < 0.001; ns nonsignificant