Fig 1.
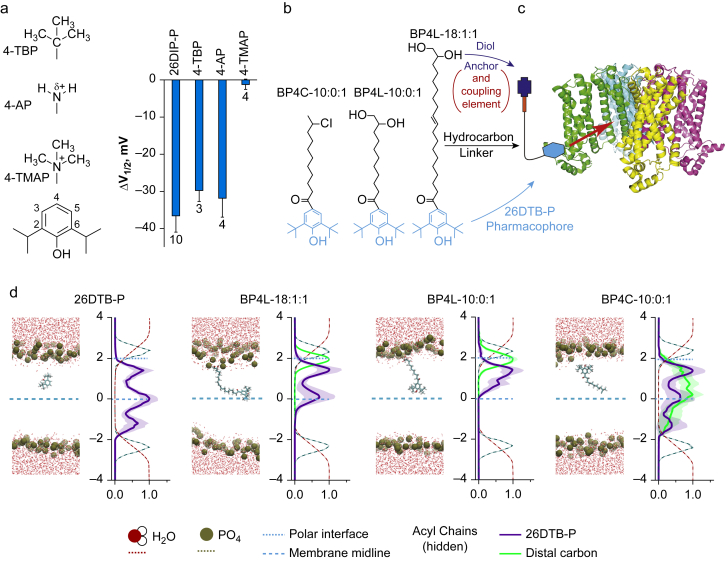
Molecular dynamic modelling demonstrates functionally tolerated para position anchors control within-membrane distribution of the 2,6-di-tert-butylphenol (26DTB-P) pharmacophore. (a) Left: structures of zero-length linker anchors used to examine the effect of charge vs volume on hyperpolarisation-activated cyclic nucleotide-regulated (HCN) 1 inverse agonist activity. Right: shift in the V1/2 (ΔV1/2) of HCN1 gating determined in two-electrode voltage clamp (TEVC) for 2,6-di-iso-propylphenol (26DIP-P also known as propofol) and 4-substituted derivatives thereof (4-TBP, 4-tert-butyl-propofol; 4-AP, 4-amino-propofol; 4-TMAP, 4-trimethylamino-propofol). 26DIP-P was at 100 μM (the concentration that is saturating with respect to HCN1 inhibition); 4-TBP, 4-AP, and 4-TMAP were each at 200 μM. The effects of 26DTB-P, 4-TBP, and 4-AP are different to the absence of effect of 4-TMAP but not different to each other (see Supplementary Table S1). (b) ChemDraw representations of an unanchored (BP4C-10:0:1) and short and long tether diol-anchored variants of 26DTB-P (BP4L-10:0:1 and BP4L-18:1:1, respectively). (c) Schematic representation of the conceived coupling between a tether-anchored alkylphenol inverse agonist and an HCN1 channel. The cyan hexagon represents the alkylphenol pharmacophore. The thin black line represents the hydrophobic tether that, in the molecules presented in (b), are saturated or partially unsaturated acyl chains. The red rectangle is a hydrophilic coupling element that links the tether to the dark blue anchor. In the molecules studied here, the coupling element and the anchor are collectively the diol unit. The channel structure is adapted from Lee and MacKinnon.48 (d) In each of the four pairs of panels, the left-hand graphic is a still image from a high-resolution molecular dynamics simulation, and the right is a normalised density distribution depicting the Z-plane occupancy within the simulation cube of the water molecules, phosphate head groups of the phospholipids, the 26DTB-P group, and the anchor–tether complex (when present) plotted with respect to the membrane midline. The behaviour of the anchor–tether complex was determined by following the distal terminal carbon (i.e., the carbon atom furthest from the pharmacophore). In each density plot, the midline of the membrane (blue dashed line) was established with respect to the distance between the phosphate peaks. Distances (in nm) are shown relative to the midline. Increasingly positive values represent the outer leaflet and extracellular medium. The location of the blue dotted line representing the polar interface was located at the maximal occupancy of the diol anchor in BP4L-18:1:1. The membrane lipid acyl chains are omitted from view for clarity.