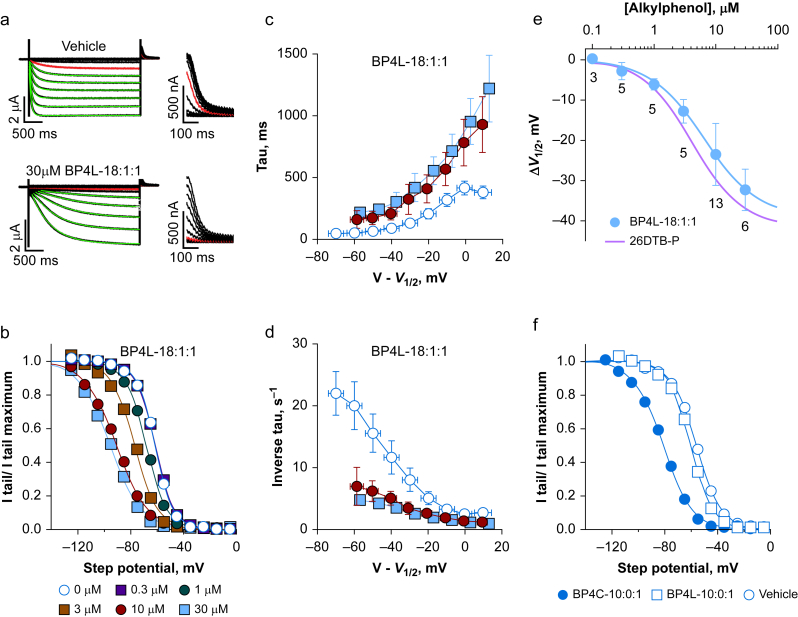
Fig 2.
BP4L-18:1:1 is a potent and efficacious hyperpolarisation-activated cyclic nucleotide-regulated (HCN) 1 inverse agonist. (a) Exemplar families of HCN1 two-electrode voltage clamp (TEVC) current traces (left, full length; right, the tail currents on expanded scales). Current traces in red were those obtained upon activation at –65 mV. The green lines are superimposed fits of a single exponential function that simultaneously optimises the time constant and the pre-exponential lag. Human HCN1 was similarly inhibited by BP4L-18:1:1 (data not shown). (b) Representative normalised tail current activation curves, each fits with the Boltzmann function. Each curve is from a different cell, but all are from the same donor frog recorded on the same day. (c, d) Activation time constants (c) and their inverse (d), plotted as a function of the step potential with respect to the V1/2 with data aggregated into 10 mV bins. Errors around the dependent variables and around the binned V–V1/2 are standard deviation (sd). Where no error is seen, it is smaller than the symbol. Data are from nine, six, and six separate recordings (control, 10 μM, and 30 μM, respectively) paired by day and donor frog. (e) Shift in the V1/2 of HCN1 gating, as determined in TEVC in the presence of BP4L-18:1:1 (filled blue circle, blue line is the superimposed fit of the Hill equation). Data are mean [sd]; the number of cells are indicated below each symbol. The potency and efficacy of HCN1 inhibition by BP4L-18:1:1 (IC50=6.4 μM; ΔV1/2 MAX=–38.9 mV; Hill coefficient=1.0) is comparable with that by free 2,6-di-tert-butylphenol (26DTB-P) (IC50=4.2 μM; ΔV1/2 MAX=–41.3 mV; Hill coefficient=1.1; the red line is the Hill fit to the shift in the V1/2 of HCN1 gating in response to 26DTB-P (see Supplementary Fig. S9). ΔV1/2 in 3, 10, and 30 μM BP4L-18:1:1 (but not 1, 0.3, or 0.1) are significantly different from vehicle controls (Supplementary Table S2) but are not different from the respective ΔV1/2 in 3, 10, and 30 μM 26DTB-P (Supplementary Table S3). (f) Representative current–voltage relationships for HCN1 Ih currents in the absence and presence of 100 μM BP4L-10:0:1 (short diol-anchored molecule) or BP4C-10:0:1 (the equally short but unanchored chlorine variant) indicate BP4L-10:0:1 is less effective than BP4C-10:0:1 (mean ΔV1/2 values [sd] were –3.6 [5.5], n=5 and –24 [6.3], n=5; –21.3 [2.4], n=5, respectively) and either the longer diol-anchored molecule, BP4L-18:1:1 (a–e) or the unanchored synthesis intermediate, BP4K-10:0:1 (mean ΔV1/2 [sd], –21.3 [2.4], n=5 at 30 μM; not shown). The ΔV1/2 values in BP4C-10:0:1 and BP4K-10:0:1 are significantly different from BP4L-10:0:1 but not from each other (Supplementary Table S4).