Fig 3.
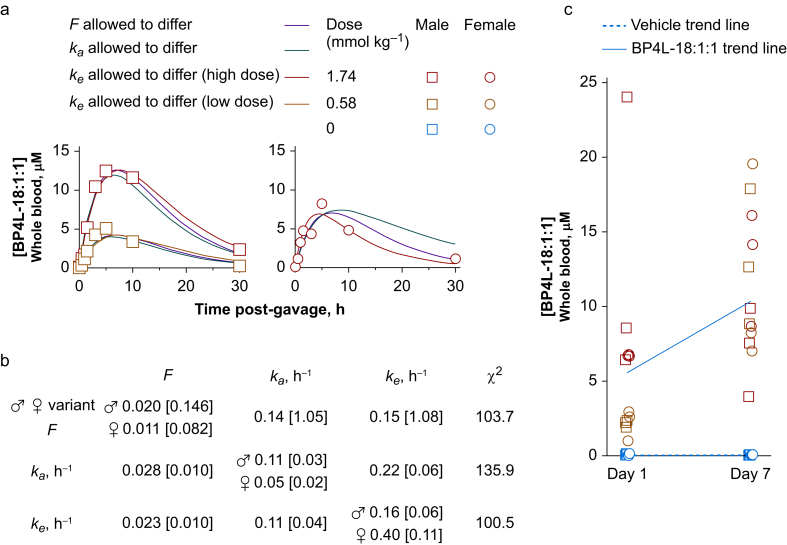
BP4L-18:1:1 is orally bioavailable following peanut oil gavage. (a) Whole blood concentrations of BP4L-18:1:1 following a single gavage of BP4L-18:1:1 with tail vein blood draws at the indicated times. Smooth lines are simultaneous fits of equation (3) to the low- and high-dose male data and the high-dose female data. Based on the approximate equilibration between major organs and blood (see Fig. 2), we assumed the volume of distribution (V) was equal to the whole-body volume (250 ml for a 250 g rat). As there is no a priori reason to attribute the observed difference in males and female peak blood concentration following the same (high) dose to any of the free parameters (F, ka, and ke), we ran fits, wherein F, ka, and ke were each allowed to be gender divergent with the other two parameters constrained to be equal between males and females. The thick dark blue and cyan lines are from the fit, where ke was variant between males and females (lowest entry in (b)). The red and green lines are fits, wherein F and ka were gender divergent, respectively. (b) Parameter estimates for fits of equation (3), as shown in (a). (c) Whole blood concentrations of BP4L-18:1:1 following a once-daily gavage with blood draws 6 h after the first gavage and 6 h after the seventh daily gavage. The trend lines are anchored at either end by the mean of low-dose and high-dose populations. Whilst the general trends suggest there is a degree of repeat dose summation (as is anticipated from the single-dose time courses in (a)), the scatter in each of the sex/dose populations suggests there is marked gavage-to-gavage variation that precludes this aspect of the current data.