Abstract
Ghee is a premium product in Southeast Asia and is prone to adulteration with vegetable oils/ fats. The main aim of the study was to develop an easy-to-use paper-based sensor to detect this adulteration. Hence, a protocol involving hexane and acetonitrile for the extraction of synthetic antioxidants from adulterated ghee and its rapid detection using DPPH was standardized. Paper-based discs impregnated with 4 mM DPPH were developed. The developed paper-based disc sensors worked well and their response time was indirectly proportional to the antioxidant concentration (0.0025–0.02%). Using the developed disc sensors, the palm oil, and sunflower oil added to cow ghee @2.5% or more, and 1% or more, respectively could be detected. The shelf life of the developed sensors was 30 and 90 days at 30 °C and 4–6 °C, respectively. In stored cow ghee samples, the response time of the sensors increased as the storage period of ghee samples increased. The cutoff limit to declare the sample of cow ghee as unadulterated was fixed to 60 min. Based on the response time of the sensor, the level of detection of vegetable oils in stored cow ghee was found to be 2.5%.
Keywords: Cow ghee, Vegetable oil, Synthetic antioxidants, Color sensor
Introduction
Ghee is the second largest dairy product after fluid milk. It is reported that ~ 35% of the milk is converted into ghee in the Indian sub-continent (Hazra et al. 2017). In India, the ghee market has witnessed substantial growth over the past few years. The market of ghee in India was valued at Indian rupees (INR) 2624 billion in 2021 (IMARC 2021). The premium nature of the product attracts unscrupulous traders to adulterate it with cheaper fats like refined vegetable oils, body fats, and vegetable fats or their concoctions (Hazra et al. 2018). The most encountered adulterant in ghee is vegetable oils because of their low cost and easy availability in comparison to ghee. To ensure a quality product to the consumer, the Government of India has specified the quality standards for ghee (FSSR 2011 and AGMARK 1981). It is also a fact that commercially available refined edible vegetable oils have low shelf life due to unsaturation and thus synthetic antioxidants or their mixtures are added to improve their oxidative stability (O’brien 2008). As per the FSSR (2011), the maximum permissible limit of synthetic antioxidants like Butylated Hydroxyanisole (BHA) and Butylhydroquinone (TBHQ) in edible vegetable oils is 200 ppm. On the other hand, no synthetic antioxidant is permitted in ghee as per ghee standards (FSSR 2011). Therefore, the presence of synthetic antioxidants in pure ghee can be used as a tracer component to detect vegetable oils adulteration in ghee. Literature suggested that the chromatographic and spectrophotometric methods have been recommended to estimate the added synthetic antioxidants in vegetable oils (Yang et al. 2002; Martinez et al. 2013; Ribeiro and Jorge 2017), but these approaches are cost-intensive, cumbersome, laborious, and time-consuming. Similarly, methods like High-performance liquid chromatography (HPLC) of sterols, Gas–Liquid Chromatography (GLC) of sterols, fatty acids, and triglycerides, and Reversed-phase thin-layer chromatography (RP-TLC) of sterols, have been reported to detect the presence of vegetable oils (palm oil, soyabean oil, coconut oil, and sunflower oil, etc.) in ghee. However, all these methods are time-consuming and cost intensive. Recently, a rapid dye-based platform test was reported to check the presence of vegetable oils in milk fat, which is based on the detection of synthetic antioxidants present in refined vegetable oils, wherein the addition of vegetable oil to the tune of 5% and more could be detected (Ramani et al. 2018). Literature also suggested that added BHA and TBHQ in soyabean oil on storing it in an oven at 60 °C for 20 days, showed some degradation (Ribeiro and Jorge 2017). Similarly, in another study, it was reported that in lard containing BHA, there was only a 10% change in the residual antioxidant level on the 42nd day of storage at 50 °C (Choi et al. 2019). In the case of butter, it is reported that the residual antioxidant content of BHA in butter after the storage of six months at −20 °C was reduced to 28.4 and 62.5 ppm from its initial value of 50 and 100 ppm, respectively. Similarly, in the case of BHT, the residual content estimated in butter after the storage of six months at −20 °C was 23.1 and 52.5 ppm, respectively (Ozturk and Cakmakci 2006). The above-said studies relating to the storage stability of added antioxidants led to draw a hypothesis that antioxidants used to enhance the shelf life of vegetable oils can get exhausted during the storage of ghee samples spiked with such oils. This may affect the sensitivity and thereby test results of the rapid platform test already reported in the literature (Ramani et al. 2018). Therefore, the main aim of the present investigation was to develop a ready-to-use paper disc-based color sensor unlike liquid dye, which has better sensitivity to detect vegetable oil addition in milk fat. In the present study, the developed paper-based disc sensor was also evaluated for its applicability in vegetable oils spiked and stored cow ghee samples. The major advantage of the developed sensor-based method over the existing methods of vegetable oil detection in ghee is its rapidity, ease of use, and freedom from procuring and making the liquid dye solution.
Materials and methods
Chemicals and reagents
2,2-diphenyl-1-picrylhydrazyl [DPPH; (C18H12N5O6)] was purchased from Merck Specialities Pvt Ltd., Mumbai, India, Methanol (HPLC grade) was purchased from Thermo Fisher Scientific India Pvt. Ltd. Mumbai; n-Hexane was purchased from Honeywell International India Pvt. Ltd.New Delhi, Acetonitrile (HPLC grade) was purchased from S.D Fine- chem. Ltd. Mumbai, India; Synthetic antioxidants viz. Butylated Hydroxyanisole (BHA), Butylhydroquinone (TBHQ) from Sigma Aldrich, St. Louis, USA.
Stock solution (0.02%) of BHA and TBHQ was prepared separately in acetonitrile and diluted further with acetonitrile to obtain the working solutions of lower concentrations (0.01, 0.005, and 0.0025%).
Procurement of pure cow ghee and adulterant vegetable oils
Pure cow ghee (PG) was collected from the Experimental Dairy (ICAR- NDRI, Karnal, Haryana, India).
The refined vegetable oils namely sunflower oil (SFO) and palm oil (PO) used in the study were purchased from the local market, Karnal, Haryana, India.
Preparation of cow ghee samples spiked with antioxidants
Cow ghee was heated at 45 °C and mixed well to ensure homogeneity in the sample. Stock solution of cow ghee containing individual antioxidants (BHA & TBHQ) @ 5% was prepared. Ghee samples with effective concentrations of antioxidants @ 0.0025, 0.005, 0.01 & 0.02% were prepared by diluting the stock ghee solution, with pure ghee devoid of any added oxidant.
Preparation and storage of PG samples spiked with vegetable oils
The adulterant vegetable oils (SFO and PO) were added individually to pure cow ghee @ 1, 2.5, 5, 7.5 & 10%. The samples were thoroughly mixed, filled in air-tight HDPE narrow-mouth bottles, and stored for nine months at room temperature (30 °C) till further analysis.
Optimization of DPPH concentration and volume for efficient visual color change in the presence of antioxidants (BHA, TBHQ)
DPPH solution of (1, 2, 3, and 4) mM conc. was prepared in methanol. Then, 1, 5, 10, 15, 20 & 25µL from DPPH solution of each conc. were pipetted out and transferred to 600µL of standard BHA and TBHQ solutions of varied conc. (0.0025, 0.005, 0.01 & 0.02%) the solution, respectively. The contents were incubated at room temperature time and the duration of color change from violet to yellow/colorless was recorded.
Optimization of conditions for the efficient extraction of antioxidants from PG samples
To achieve this target, molten cow ghee was spiked with antioxidants (BHA & TBHQ) @ 5% to get the stock solution of the antioxidant-spiked cow ghee sample. The above-said stock solution was then diluted with pure cow ghee to prepare samples with effective concentrations of antioxidants @ 0.0025, 0.005, 0.01 & 0.02%. To optimize efficient extraction of antioxidants from cow ghee samples spiked with varied concentrations of antioxidants, two approaches were tried i.e. single step and two-step extraction approaches coupled with centrifugation (2000 rpm/5 min) and gravitational separation (5–10 min) of acetonitrile layer. In the case of single-step extraction, 1.0 ml of molten ghee was mixed with 1.0 ml of hexane followed by the addition of 1.0 ml of acetonitrile. The contents of the tube were then centrifuged (2000 rpm/5 min) or allowed to stand undisturbed for gravitational separation (5–10 min). The clear acetonitrile layer was collected in 2 ml micro-centrifuge tubes.
In the case of the two-step approach, the first extraction was similar to the process mentioned above in the single-step approach. In the second extraction, an additional step of acetonitrile extraction was performed using 1.0 ml of acetonitrile followed by centrifugation (2000 rpm/5 min) or gravitational separation (5–10 min). The clear acetonitrile layer collected in the second extraction was mixed with the earlier one.
Optimized protocol to extract antioxidants from PG samples
To ensure the complete extraction of the synthetic antioxidants from cow ghee, 1.0 ml of antioxidant spiked ghee sample was mixed with 1.0 ml of hexane and then 1.0 ml acetonitrile followed by vortexing for 30 s and then centrifugation at 2000 rpm/5 min. The upper layer was collected and the extraction step with an additional 1.0 ml of acetonitrile was repeated. The upper layer collected in the previous step was then mixed with the upper layer collected in the second extraction. This extract was then used in the test to detect vegetable oils' presence in ghee.
Fabrication of paper-based sensor discs
In this set of experiments, Whatman filter paper 4 was cut into small circular discs (0.5 cm diameter) by using a regular paper punching machine. To optimize the loading capacity of the paper discs, varied volumes (1, 5, 10, 15, 20, and 25µL) of 4 mM methanolic DPPH were dispensed onto the discs followed by drying at ambient temperature (30 °C) to obtain dried discs.
Shelf life of developed paper-based color sensor discs
The prepared disc color sensor i.e., DPPH-impregnated discs were packed in HDPE amber-colored bottles and then stored at refrigerated (4–6 °C) and ambient temperature (30 °C) for up to six months. These were then evaluated periodically to evaluate their shelf life, wherein their performance was seen in antioxidant solutions. In this experiment, 600 μL of freshly prepared standard antioxidant BHA, BHT (0.0025, 0.005, 0.01 & 0.02%) solutions were used to evaluate the efficiency of these sensors by observing the time duration of color change from violet to colorless or yellow. The endpoint of the non-suitability of the developed sensor was either no color change (original color of the sensor i.e. violet in antioxidant solution) or prolonged time to change the color after adding the sensor to standard antioxidant solutions of the above-mentioned concentrations.
Evaluation of the performance of the developed sensor in vegetable oils spiked ghee samples stored at 30 °C:
Cow ghee samples spiked with vegetable oils (Palm oil and Sunflower oil) were stored in HDPE plastic bottles at 30 °C. Antioxidants were extracted as per the optimized protocol stated in the methodology section every 15 days intervals up to 9 months. The developed paper-based disc sensors were then evaluated based on duration of response time i.e. time taken to show a change in color from violet to color less or yellow in the acetonitrile extract obtained from the vegetable oils spiked ghee samples.
Statistical analysis
The data obtained in the present study were subjected to one-way and two-way Analysis of variance (ANOVA) to check the significant difference in the means via Duncan’s Multiple Comparison test performed at a 95% confidence interval with IBM SPSS Statistics (version 20). The results were expressed as mean with standard deviation.
Results and discussion
Effect of DPPH concentration and volume on the visual intensity of color in the presence of antioxidants
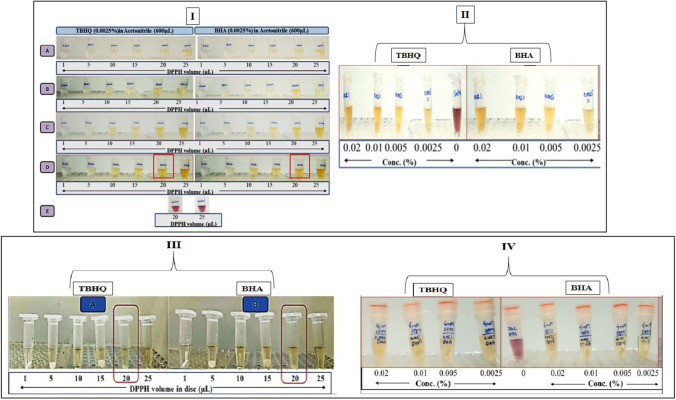
Literature suggested that methanol had been used as a solvent for DPPH solution in paper-based techniques (Akar et al. 2017; Sirivibulkovit et al. 2018; Kuswandi et al. 2020). Hence the solution of DPPH in methanol was also tried in the study. To optimize the DPPH concentration and volume, a constant volume (600 µL) of antioxidant solution (0.0025%) in acetonitrile was used. It is evident from the results [Fig. 1(I)] that in control (E) i.e., acetonitrile without any antioxidant there was the formation of violet color on the addition of (20 and 25 µL) DPPH solution of 4 mM conc. It is also evident from the results [(Fig. 1(I)] that a sharp color change from violet to yellow was observed in the antioxidant-containing tubes wherein 20 & 25 µL of DPPH solution of 4 mM concentration was added. However, in the case of tubes having the same concentration of antioxidants, but the addition of 20 & 25 µL of 2 and 3 mM DPPH, the color change was not very sharp and the visual color intensity of yellow color was less, unlike 4 mM DPPH solution. This gave the clear-cut indication that either of the volumes i.e. 20 or 25 µL of 4 mM DPPH solution can be used to bring a perceptible shift in color from violet to yellow. This was further confirmed from the findings [Fig. 1(II)] that 20 µL of 4 mM DPPH was sufficient to show a color change from violet to yellow or disappearance of violet color in 600 µL of antioxidant solution of studied concentrations (0.0025–0.02%) in acetonitrile. Hence, the use of 20 µL of 4 mM DPPH solution was continued in the subsequent experiments and the response time required to shift the color from violet to yellow or the disappearance of the violet color of DPPH solution in the presence of different antioxidants was studied, whose findings have been depicted in Table 1.
Fig. 1.
[I] Color change in DPPH solution in the presence of antioxidants (BHA and TBHQ) solution in acetonitrile: A (1 mM DPPH), B (2 mM DPPH), C (3 mM DPPH) and D (4 mM DPPH), E: Control (4 mM DPPH) in the absence of antioxidants [II] Color change in DPPH solution (TBHQ) and volume (20µL) in the presence of antioxidants solutions of varied concentration [III]: Change in visual color intensity in TBHQ & BHA solutions of 0.0025% conc in acetonitrile on adding paper discs prepared by impregnating DPPH of varied conc. [IV] Effect of antioxidants’ concentration on the response of developed disc-based sensor
Table 1.
Effect of antioxidants concentration on the duration of time to change color from violet to yellow in 4 mM DPPH solution and developed disc-based sensor
| Concentration of antioxidant added in ghee (%) | 4 mM DPPH solution | Developed DPPH impregnated disc based sensor | ||
|---|---|---|---|---|
| BHA (600 µL) | TBHQ (600 µL) | BHA (~ 2000µL) | TBHQ (~ 2000µL) | |
| Time (min)- | Time (min) | |||
| 0 | > 4400 | > 4400 | > 4400 | > 4400 |
| 0.02 | 3.00 ± 0.50A | 1.00 ± 0.25A | 3.00 ± 0.50A | 1.00 ± 0.00A |
| 0.01 | 6.33 ± 0.50B | 2.00 ± 0.50B | 6.33 ± 0.53B | 2.00 ± 0.025B |
| 0.005 | 13.00 ± 1.0C | 3.00 ± 0.50C | 12.67 ± 1.33C | 3.00 ± 0.50C |
| 0.0025 | 26.77 ± 1.5D | 5.83 ± 1.00D | 27.00 ± 1.58D | 6.33 ± 0.50D |
Values are represented as Mean ± SD, n = 3; A−D–different superscript column-wise differ significantly (p < 0.05)
It is evident from the data (Table 1) that the response time (Min) to change color in the studied conc. (0.02, 0.01, 0.005, and 0.0025) % of BHA and TBHQ was (3.00 ± 0.50, 6.33 ± 0.50, 13.00 ± 1.00, 26.77 ± 1.50) and (1.00 ± 0.25, 2.00 ± 0.50, 3.00 ± 0.50, 5.83 ± 1.00), respectively. The perusal of the data (Table 1) also revealed that there was a considerable difference in the duration of color change between BHA and TBHQ. In the case of the TBHQ solution, the color change was fast as compared to the similar concentration of the BHA solution. The clear reason for this difference could not be ascertained, because recent literature suggested that the antioxidant potential of BHA was almost equal to TBHQ (Thbayh and Fiser 2022). The response time observed in the control tube i.e. only acetonitrile without any synthetic antioxidant was ≥ 4400 min or 73 h.
Effect of centrifugation and single/double extraction on the time required to change the color of DPPH solution
To optimize the conditions for efficient extraction of added antioxidants from ghee, the main aim was to determine the time duration of a color change from violet to yellow or/ colorless in the antioxidant extract. To achieve this the pre-requisite was to obtain the clear extract from the antioxidant spiked ghee samples. Therefore, in the preliminary trials, it was experienced that the extract obtained without centrifugation i.e. gravitational separation, was not clear and had many air bubbles, hence centrifugation (2000 rpm/5 min) was preferred over gravitational separation (5–10 min). The acetonitrile layer containing extracted antioxidants was used in the subsequent experiments of antioxidant extraction optimization.
It is evident from the results (Table 2), that the time duration to observe the color change from violet to yellow in 4 mM DPPH solution varied from 1.0 to 3.0 min and 1.0–1.83 min, in BHA and TBHQ extracted from respective spiked ghee samples using single step extraction. The perusal of the data also revealed that the variation in time duration of color change was indirectly proportional to the concentration of BHA and TBHQ spiked in the ghee samples. As the concentration of added BHA or TBHQ in ghee was decreased the time duration of the perceptible change in color of DPPH solution from violet to yellow/colorless was increased. It is also evident from the results that there was a significant (p ≤ 0.05) difference in the increase of response time of the sensor discs as the concentration of the added antioxidants was decreased. However, the interesting observation was that the time duration of color change was too short in a single-step extraction approach as compared to the duration observed in the corresponding concentration of the standard BHA and TBHQ in acetonitrile (Table 1). This difference in the time may be attributed to the fact that the antioxidant extracted from 1.0 ml of antioxidant-spiked cow ghee has been concentrated in 1.0 ml of acetonitrile and due to this concentration factor this deviation was observed.
Table 2.
Effect of single/two-step antioxidant extraction from ghee on the time of color change of DPPH solution
| Concentration of antioxidant added in ghee (%) | Single step extraction | Two- step extraction | ||
|---|---|---|---|---|
| BHA (600 µL) | TBHQ (600 µL) | BHA (~ 2000µL) | TBHQ (~ 2000µL) | |
| Time (min) | Time (min) | |||
| 0 | 86 ± 4.00E | 82 ± 3.00E | 240 ± 7E | 243 ± 9E |
| 0.02 | 1.00 ± 0.25A | 1.00 ± 0.25A | 3.00 ± 0.25A | 1.00A |
| 0.01 | 1.83 ± 0.29B | 1.00 ± 0.29A | 6.00 ± 0.50B | 2.00 ± 0.50B |
| 0.005 | 2.50 ± 0.5C | 1.00 ± 0.50A | 12.66 ± 1.00C | 3.00 ± 0.50C |
| 0.0025 | 3.00 ± 0.50D | 1.83 ± 0.50B | 27.00 ± 1.50D | 6.00 ± 0.50D |
Values are represented as Mean ± SD, n = 3; A−E–different superscript column-wise differ significantly (p < 0.05)
The perusal of the data (Table 2), also revealed that in the two-step extraction approach, the observed time of color change in extracted antioxidants on the addition of 20 µL of 4 mM DPPH solution was 3–27 min depending upon the concentration of the antioxidant added to ghee samples. In this case, the time recorded to show a color change was similar to the time observed (Table 1) in the case of the corresponding concentration of pure BHA and TBHQ solutions in acetonitrile. This can be attributed to the dilution effect of about a two-fold increase in the volume of acetonitrile used for antioxidant extraction. The two-step extraction might have nullified the effect of concentration as exhibited in terms of the short duration of the color change of the DPPH solution in single-step extraction.
Since the time duration of color change was almost similar to the duration observed in pure antioxidant solutions of the corresponding conc., hence, the two-step extraction was preferred over single-step extraction.
Optimization of the volume of DPPH to be impregnated on paper-based discs
Effect of impregnated DPPH concentration on visual color change and time duration to change the color of antioxidants solution
The concentration of DPPH to be impregnated on paper discs was optimized by dispensing different volumes (1, 5, 10, 15, 20, and 25 µL) of DPPH solution of 4 mM concentration on paper discs. These developed discs were then added into 600 µL 0.0025% solution of antioxidants i.e., TBHQ and BHA followed by the recording of time duration of perceptible color change in the solution. It is evident from the result [Fig. 1(III)] that disc prepared by adding 20 & 25µL of 4 mM DPPH solution resulted in a sharp color change from violet to yellow in the tubes containing 600 µL of 0.0025% solution of antioxidants. Since the DPPH-impregnated paper disc by adding 20µL of 4 mM DPPH was enough to show a perceptible color change in the antioxidant solutions [Fig. 1(IV)] hence, the above-said volume was finalized to prepare the paper-based disc sensors.
The above-mentioned developed paper-based discs were used to determine the time duration of color change from violet to yellow in the antioxidant solutions of varied concentrations. It is evident from the results (Table 1) that the response time of the disc to bring a color change in the control sample was more than 73 h vis′-a-vis′ ~ 3.0 min in 0.02% acetonitrile solution of BHA. As the concentration of BHA in acetonitrile solution was decreased the time duration to show a color change on adding the prepared disc was increased. A similar trend was observed in the case of TBHQ solutions of varied concentrations as evident from the data (Table 1). The perusal of the data also revealed that the response time of the developed paper-based sensor discs was increased significantly (P < 0.05) as the concentration of antioxidants was decreased in the solution.
Effect of storage temperature (30 °C) and 4–6 °C) on the stability of paper-based sensor discs
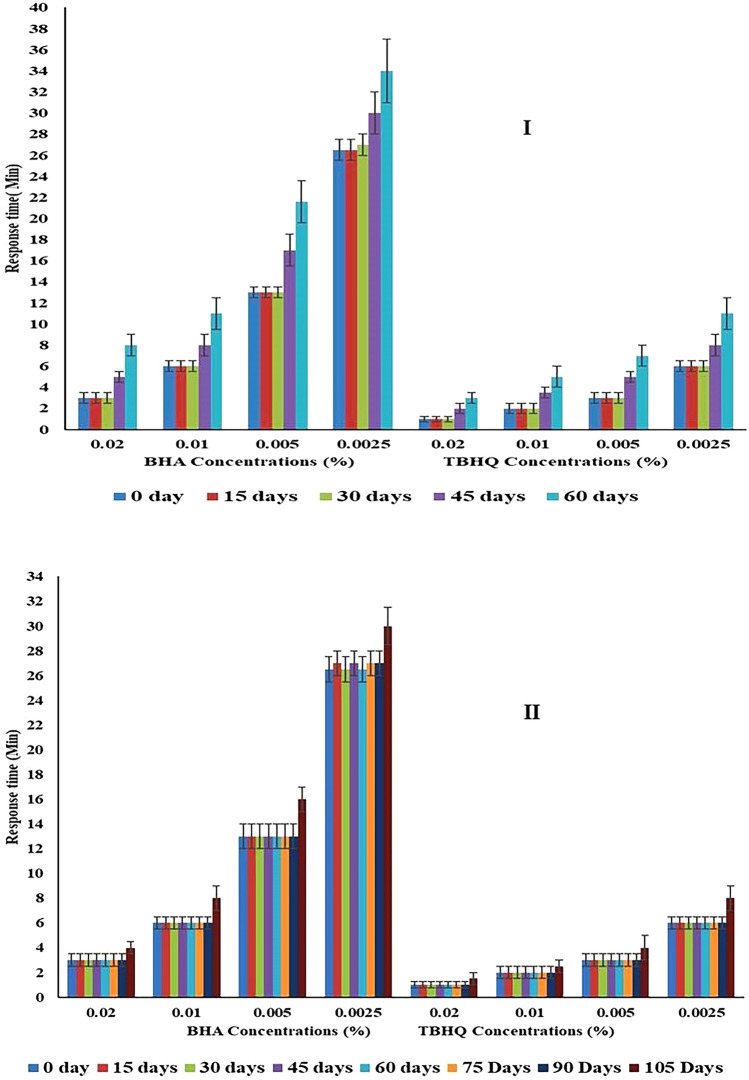
It is evident from the bar diagram [Fig. 2(I)] that the response time to show a visible change in color from violet to yellowish or colorless in the acetonitrile solution of studied concentrations of antioxidants (BHA and TBHQ) was almost the same to the response time of freshly prepared discs till the 30th day of storage at ambient temperature (30 °C). The results with respect to the storage stability of DPPH-impregnated paper-based discs were in line with the earlier findings (Sirivibulkovit et al. 2018) that the paper-based DPPH device wrapped with aluminum foil was stable for up to 30 days at room temperature (26–31 °C).
Fig. 2.
Effect of storage on the response time of prepared disc sensor at [I] (30 °C) and [II] 4–6 °C
The bar diagram [Fig. 2(II)] clearly showed that the response time of the developed paper-based sensor disc for the studied concentration of antioxidants (BHA and TBHQ) was comparable to freshly prepared discs till the 90th days of storage at refrigerated temperature (4–6 °C). Thereafter, a significant (p ≤ 0.05) increase in the response time of the stored sensors was observed for all the studied antioxidants and their corresponding concentrations. It was concluded that disc sensors were found to have better storage stability or shelf life both at room as well as refrigerated temperatures.
Effect of vegetable oils (PO, SFO) spiking in cow ghee (PG) and storage temperature (30 °C) of samples on the response of DPPH-impregnated paper-based sensor discs
The response time of DPPH impregnated paper-based disc sensor was determined and expressed in min. It is evident from the results (Table 3) that in stored PO samples the response time of DPPH-impregnated paper-based disc sensor remained unaltered till 135 days of storage at ambient temperature. It is also evident from the data (Table 3) that the response time of the sensor discs in the case of palm oil was 0.50 min. on 0 days of storage. However, in the case of the corresponding sample, the response time of the sensor disc was increased to 1.0 min. at the end of storage of 9 months (270 days). It was also observed that the response time of the sensor disc in control PG significantly increased (P < 0.05) from 250.00 ± 5.00 min on 0 days to 416.00 ± 5.29 min. at the end of 9 months (270 days). It is also evident from the results that the response time in all the samples spiked with PO up to 2.5% was lower than the response time of the sensor in PG. On the contrary, in the case of ghee samples spiked with PO @1% the response time of the sensor was 246.67 min. on 270th day of storage, which was very close to the response time of the sensor observed on 0th day in PG samples. These findings led to the conclusion that on account of storage, there may be the degradation of the added antioxidant/ utilization of the added antioxidant to counter the oxidation process. This exhaustion of the antioxidant may be responsible for the increase in the response time of the sensor in stored ghee samples as well as stored PO-spiked ghee samples.
Table 3.
Response time of DPPH impregnated paper-based disc sensor in stored PG, PO and PO spiked PG samples at ambient temperature (30 °C)
| Storage Days | Response time (min) | ||||||
|---|---|---|---|---|---|---|---|
| PG | PO | Si | Sii | Siii | Siv | Sv | |
| 0 | 250.00 ± 5.00aD | 0.50aA | 3.00 ± 0.50aA | 5.00 ± 0.50aA | 8.67 ± 0.58aA | 20.33 ± 0.58aB | 143.33 ± 5.55aC |
| 15 | 250.00 ± 5.00aE | 0.50aA | 3.00 ± 0.50aAB | 5.00 ± 0.50aAB | 9.33 ± 0.58aB | 20.67 ± 0.58aC | 153.33 ± 2.89abD |
| 30 | 265.00 ± 5.00bF | 0.50aA | 3.00 ± 0.50abAB | 5.67 ± 0.58abB | 10.33 ± 0.58bC | 29.67 ± 0.58bD | 158.33 ± 2.89abE |
| 45 | 276.67 ± 5.77cF | 0.50aA | 3.00 ± 0.50abcAB | 5.33 ± 0.58abcB | 10.67 ± 0.58bC | 34.00 ± 2.00cD | 166.67 ± 2.89bcE |
| 60 | 285.00 ± 5.00cdF | 0.50aA | 3.33 ± 0.58abcdAB | 5.67 ± 0.58abcB | 11.67 ± 0.58cC | 41.00 ± 1.73dD | 170.00 ± 5.00bcdE |
| 75 | 288.33 ± 2.89dF | 0.50aA | 3.67 ± 0.58abcdB | 5.67 ± 0.58abcB | 11.67 ± 0.58cC | 42.00 ± 1.73deD | 178.33 ± 2.89cdE |
| 90 | 293.33 ± 5.77deF | 0.50aA | 4.00 ± 0.50abcdAB | 6.00 ± 0.50bcdB | 11.67 ± 0.58cC | 44.33 ± 2.52eD | 188.33 ± 2.89deE |
| 105 | 300.00 ± 5.00eF | 0.50aA | 4.00 ± 0.50abcdAB | 6.33 ± 0.58cdB | 12.33 ± 0.58cdC | 48.00 ± 1.00fD | 198.33 ± 2.89efE |
| 120 | 300.00 ± 5.00fE | 0.50aA | 4.00 ± 0.50abcdA | 6.33 ± 0.58cdAB | 12.33 ± 0.58cdB | 49.67 ± 1.58fC | 205.00 ± 5.00efD |
| 135 | 325.00 ± 5.00gF | 0.50aA | 4.00 ± 0.50abcdAB | 6.67 ± 0.58 dB | 12.33 ± 0.58cdC | 52.00 ± 1.00gD | 211.67 ± 2.89fgE |
| 150 | 337.67 ± 2.52hF | 1.00aA | 4.00 ± 0.50abcdB | 6.67 ± 0.58 dB | 12.33 ± 0.58cdC | 54.00 ± 1.00ghD | 216.33 ± 4.04fghE |
| 165 | 353.33 ± 5.77iD | 1.00aA | 4.67 ± 0.58bcdA | 7.67 ± 0.58eA | 12.67 ± 0.58cdA | 56.00 ± 1.00hiB | 220.33 ± 4.81ghiC |
| 180 | 360.00 ± 5.00iF | 1.00aA | 4.67 ± 0.58cdAB | 8.00 ± 0.50eB | 13.00 ± 1.00deC | 57.67 ± 1.58ijD | 225.00 ± 5.00hijE |
| 195 | 373.33 ± 2.89jG | 1.00aA | 4.67 ± 0.58cdB | 8.00 ± 0.50eC | 13.33 ± 1.58defD | 59.00 ± 1.00jkE | 228.67 ± 5.89hijF |
| 210 | 383.33 ± 2.89kG | 1.00aA | 5.00 ± 0.50 dB | 8.00 ± 0.50eC | 13.67 ± 1.58efgD | 60.33 ± 1.58klE | 230.33 ± 5.15ijkF |
| 225 | 391.67 ± 2.89klG | 1.00aA | 5.00 ± 0.50 dB | 8.00 ± 0.50eC | 14.00 ± 1.00ghiD | 60.33 ± 1.15klE | 233.00 ± 5.73jkF |
| 240 | 395.00 ± 5.00lmF | 1.00aA | 5.00 ± 0.50 dB | 8.00 ± 0.50eB | 14.33 ± 1.58hiC | 61.33 ± 1.15klD | 236.33 ± 5.15jkE |
| 255 | 404.00 ± 5.29mF | 1.00aA | 5.00 ± 0.50dAB | 8.00 ± 1.00eB | 14.67 ± 1.58ijC | 62.00 ± 1.73lD | 241.67 ± 5.89jkE |
| 270 | 416.00 ± 5.29nF | 1.00aA | 5.00 ± 0.50dAB | 8.00 ± 1.00eB | 15.33 ± 1.58jC | 65.00 ± 2.00mD | 246.67 ± 5.89kE |
Values are represented as Mean ± SD, n = 3; a, b & A, B–different superscript row-wise and column-wise, respectively differ significantly (p < 0.05)
PG: Pure Ghee; PO: Palm Oil; Si: PG spiked with PO @ 10%; Sii: PG spiked with PO @ 7.5%; Siii: PG spiked with PO @ 5%; Siv: PG spiked with PO @ 2.5%; Sv: PG spiked with PO @ 1%
The perusal of the data (Table 4) revealed that in the stored SFO sample, the response time of the DPPH-impregnated paper-based sensor disc remained unaltered till 120 days of storage at 37 °C. It is also evident from the data (Table 4) that in the case of SFO, the response time noted was 0.50 min on zero days of storage. It was also observed that in spiked ghee samples, the response time significantly increased (P < 0.05) and was found to be more than the pure SFO. This could be attributed to the dilution of the antioxidant present in SFO when it was spiked in pure ghee samples. As the storage period progressed an increase in the response time of the sensor disc was observed in all the samples including pure ghee as well as SFO. The response time of the sensor disc increased significantly (P < 0.05) from 250.00 ± 5.00 on 0 days to 416.00 ± 5.29 at the end of 9 months (270 days) in PG samples. Similarly, the response time of SFO was increased to 1.33 on 270 days of storage. In the ghee samples spiked with SFO @ 2.5% and subsequently stored, the response time of the sensor disc noted on 270th day was 27.67 min. This response time was very less as compared to the pure ghee sample on zero-day. As the level of adulteration was further decreased to 1%, the response time was increased to 156 min. on 270th day of storage. Though the response time was still less than the response time in control ghee on zero days, however, the safest level of detection considered was 2.5% as the response time was less than 30 min.
Table 4.
Response time of DPPH impregnated paper-based disc sensor in stored PG, SFO and SFO spiked PG samples at ambient temperature (30 °C)
| Storage Days | Response time (min) | ||||||
|---|---|---|---|---|---|---|---|
| PG | SFO | Ti | Tii | Tiii | Tiv | Tv | |
| 0 | 250.00 ± 5.00aE | 0.50aA | 3.00 ± 0.25aAB | 5.00 ± 0.50aAB | 9.33 ± 0.58aB | 19.00 ± 1.0aC | 60.67 ± 1.15aD |
| 15 | 250.00 ± 5.00aE | 0.50aA | 3.00 ± 0.25aAB | 5.00 ± 0.50aAB | 9.67 ± 0.58aB | 19.00 ± 1.0aC | 63.00 ± 1.73aD |
| 30 | 265.00 ± 5.00bF | 0.50aA | 3.00 ± 0.25aAB | 6.33 ± 0.58bBC | 9.67 ± 0.58abC | 19.67 ± 0.58aD | 69.00 ± 1.73bE |
| 45 | 276.67 ± 5.77cF | 0.50aA | 3.67 ± 0.58abAB | 6.33 ± 0.58bBC | 9.67 ± 0.58abC | 19.00 ± 1.00aD | 74.33 ± 1.15cE |
| 60 | 285.00 ± 5.00cdF | 0.50aA | 3.67 ± 0.58abAB | 6.33 ± 0.58bBC | 9.67 ± 0.58abC | 22.33 ± 1.53aD | 78.00 ± 1.73dE |
| 75 | 288.33 ± 2.89dG | 0.50aA | 3.67 ± 0.58abB | 6.67 ± 0.58bC | 9.67 ± 0.58abD | 20.00 ± 1.0bE | 83.00 ± 1.73eF |
| 90 | 293.33 ± 5.77de | 0.50aA | 4.00 ± 0.50bcAB | 6.67 ± 0.58bcBC | 10.00 ± 0.50abC | 22.33 ± 0.58bD | 88.67 ± 1.15fE |
| 105 | 300.00 ± 5.00eF | 0.50aA | 4.00 ± 0.50bcAB | 6.67 ± 0.58bcB | 10.67 ± 0.58abcC | 23.33 ± 0.58bcD | 93.33 ± 2.89gE |
| 120 | 300.00 ± 5.00fF | 0.50aA | 4.00 ± 0.50bcAB | 6.67 ± 0.58bcAB | 10.67 ± 0.58abcB | 23.33 ± 0.58bcC | 95.67 ± 2.15gD |
| 135 | 325.00 ± 5.00gE | 0.67 ± 0.29aA | 4.33 ± 0.58bcdAB | 7.33 ± 0.58bcdBC | 11.00 ± 1.00bcC | 23.33 ± 1.53bcD | 99.00 ± 2.73hE |
| 150 | 337.67 ± 2.52hFG | 0.67 ± 0.29aA | 4.33 ± 0.58bcdB | 7.33 ± 0.58bcdC | 11.00 ± 1.00bcD | 24.33 ± 1.58cdE | 103.67 ± 2.15iF |
| 165 | 353.33 ± 5.77iF | 1.00 ± 0.25bA | 4.67 ± 0.58cdeAB | 7.67 ± 0.58cdeBC | 11.67 ± 1.53cdC | 24.67 ± 1.58cdD | 108.00 ± 2.73jE |
| 180 | 360.00 ± 5.00iF | 1.00 ± 0.25bA | 4.67 ± 0.58cdeB | 7.67 ± 0.58cdeB | 12.33 ± 1.15deC | 25.00 ± 1.50deD | 113.00 ± 2.73kE |
| 195 | 373.33 ± 2.89jG | 1.00 ± 0.25bA | 4.67 ± 0.58cdeB | 7.67 ± 0.58cdeC | 12.67 ± 0.58deD | 25.33 ± 1.58defE | 118.67 ± 2.15lF |
| 210 | 383.33 ± 2.89kG | 1.17 ± 0.29bA | 5.00 ± 0.50deB | 7.67 ± 0.58cdeC | 13.00 ± 0.50eD | 25.67 ± 1.58defE | 124.33 ± 2.58mF |
| 225 | 391.67 ± 2.89klG | 1.17 ± 0.29bA | 5.00 ± 0.50deB | 8.00 ± 1.00deC | 13.33 ± 0.58eD | 26.33 ± 1.58efgE | 131.33 ± 2.15nF |
| 240 | 395.00 ± 5.00lmF | 1.33 ± 0.29bA | 5.33 ± 0.58eB | 8.33 ± 0.58deB | 13.67 ± 0.58eC | 26.67 ± 1.58fgD | 140.67 ± 2.15oE |
| 255 | 404.00 ± 5.29mF | 1.33 ± 0.29bA | 5.33 ± 0.58eB | 8.67 ± 0.58eB | 13.67 ± 0.58eC | 27.33 ± 1.58hD | 148.33 ± 2.15pE |
| 270 | 416.00 ± 5.29nF | 1.33 ± 0.29bA | 5.33 ± 0.58eB | 8.67 ± 0.58eB | 13.67 ± 0.58eC | 27.67 ± 1.58hD | 156.33 ± 2.15qE |
Values are represented as Mean ± SD, n = 3; a, b & A, B–different superscript row-wise and column-wise, respectively differ significantly (p < 0.05)
PG: Pure Ghee; SFO: Sunflower Oil; Ti: PG spiked with SFO @ 10%; Tii: PG spiked with SFO @ 7.5%; Tiii: PG spiked with SFO @ 5%; Tiv: PG spiked with SFO @ 2.5%; Tv: PG spiked with SFO @ 1%
This increase in the response time to change the color can be attributed to the possibility of exhaustion of some amount of the antioxidants during storage. The above-said possibility is supported by the earlier studies (Ribeiro and Jorge 2017), which observed degradation of added BHA and TBHQ in soybean oil on storing in an oven at 60 °C for 20 days. Similarly, in another study, it was reported that in lard containing BHA, there was only a 10% change in the residual antioxidant level on 42nd day of storage at 50 °C (Choi et al. 2019).
Conclusion
In this study, a DPPH-impregnated paper-based disc sensor was developed. A two-step extraction approach of antioxidants from suspected samples of ghee was found to be effective in detecting the added vegetable oils @ 1% or above. The developed sensor could check the studied vegetable oils' addition to ghee @ 2.5%. The developed sensor could also detect these added vegetable oils in stored cow ghee samples. This approach of using the developed ready-to-use sensor disc had the benefit of ease of use without the hassle of preparation of liquid dye.
Acknowledgements
Authors are thankful to the Director of NDRI, Karnal for providing facilities to carry out the research and acknowledge the financial help provided by the National Dairy Research Institute, Karnal, Haryana, India.
Abbreviations
- BHA
Butylated hydroxyanisole
- Conc.
Concentration
- DPPH
2,2-Diphenyl-1-picrylhydrazyl
- TBHQ
Butylhydroquinone
- PG
Pure cow ghee
- SFO
Sunflower oil
- PO
Palm oil
Author contributions
YKN: Experimental work; investigation; methodology; statistical analysis. VS: Conceptualization; data curation; investigation; methodology; supervision; writing original draft. SA: Conceptualization; methodology; resources; editing. RS: resources; editing.
Funding
The project was a student project, which was supported by the Institute.
Data availability
The authors declare that the data supporting the findings of this study are available within the article.
Declarations
Conflict of interest
He authors have neither any conflicts of interest nor competing interest to declare.
Consent for publication
The work is a bonafied work carried out under a Doctoral program and the authors give their consent to publish the work and images included in the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yugal Kishor Naik, Email: yugalnaik7@gmail.com.
Vivek Sharma, Email: vivek.vishk12000@gmail.com, Email: vivek.sharma@icar.gov.in.
Sumit Arora, Email: sumitak123@gmail.com.
Raman Seth, Email: ramanseth123@yahoo.co.in.
References
- AGMARK (1981) Ghee Grading and Marking Rules, 1938 (as amended). Government of India, Ministry of Food and Agriculture, Department of Agriculture, New Delhi
- Akar Z, Küçük M, Doğan H. A new colorimetric DPPH scavenging activity method with no need for a spectrophotometer applied on synthetic and natural antioxidants and medicinal herbs. J Enzyme Inhib Med Chem. 2017;32:640–647. doi: 10.1080/14756366.2017.1284068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Jang GW, Choi SI, Jung TD, Cho BY, Sim WS, Lee OH. Development and validation of an analytical method for carnosol, carnosic acid and rosmarinic acid in food matrices and evaluation of the antioxidant activity of rosemary extract as a food additive. Antioxidants. 2019;8:76. doi: 10.3390/antiox8030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FSSR (2011) File no.1 94/FSSAI/SP/ (labelling) Food Safety and Standards Authority of India (Ministry of Health and Family Welfare). FDA Bhavan, Kotla Road New Delhi-110002
- Hazra T, Sharma V, Saha Pand Arora S. Triglyceride profiling of ghee using gas chromatography. Int J Chemi Stud. 2017;5:1598–1601. [Google Scholar]
- Hazra T, Sharma V, Sharma R, Arora S. Triglyceride profiling of ghee containing goat tallow and admixture of soy oil and goat tallow for detecting such an adulteration. Ind J Dairy Sci. 2018;71:176–182. [Google Scholar]
- IMARC (2021) Ghee market in India: industry trends, share, size, growth, opportunity and forecast 2022–2027. https://www.marketresearch.com/IMARC-v3797/Ghee-India-Trends-Share-Size-31292447/. Accessed 17th June 2022
- Kuswandi B, Fantoni M, Hidayat MA, Ningsih IY. Paper microzone plate based on DPPH as a simple colorimetric assay of the total antioxidant content of herbal extracts. J Food Sci Technol. 2020;57:1971–1976. doi: 10.1007/s13197-020-04378-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez ML, Penci MC, Ixtaina V, Ribotta PD, Maestri D. Effect of natural and synthetic antioxidants on the oxidative stability of walnut oil under different storage conditions. LWT-Food Sci Technol. 2013;51:44–50. doi: 10.1016/j.lwt.2012.10.021. [DOI] [Google Scholar]
- O'brien R D (2008) Fats and oils: formulating and processing for applications. CRC press
- Ozturk S, Cakmakci S. The effect of antioxidants on butter in relation to storage temperature and duration. Eur J Lipid Sci Technol. 2006;108:951–959. doi: 10.1002/ejlt.200600089. [DOI] [Google Scholar]
- Ramani A, Hazra T, Sudheendra CVK, Hariyani A, Prasad S, Ramani VM. Comparative appraisal of ghee and palm oil adulterated ghee on the basis of chromogenic test. Int J Curr Microbiol Appl Sci. 2018;7:623–627. doi: 10.20546/ijcmas.2018.712.077. [DOI] [Google Scholar]
- Ribeiro EF, Jorge N. Oxidative stability of soybean oil added to coffee husk extract (Coffea arabica L.) under accelerated storage conditions. Food Sci Technol. 2017;37:5–10. doi: 10.1590/1678-457x.06117. [DOI] [Google Scholar]
- Sirivibulkovit K, Nouanthavong S, Sameenoi Y. DPPH based assay for antioxidant activity analysis. Anal Sci. 2018;34:795–800. doi: 10.2116/analsci.18P014. [DOI] [PubMed] [Google Scholar]
- Thbayh DK, Fiser B. Computational study of synthetic and natural polymer additives-=antioxidant potential of BHA, TBHQ, BHT and curcumin. Polym Degrad Stab. 2022;201:109979. doi: 10.1016/j.polymdegradstab.2022.109979. [DOI] [Google Scholar]
- Yang MH, Lin HJ, Choong YM. A rapid gas chromatographic method for direct determination of BHA, BHT and TBHQ in edible oils and fats. Food Res Int. 2002;35:627–633. doi: 10.1016/S0963-9969(01)00164-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article.