Figure. 2: Electrostatic interactions between MK-6240 and PHFs and correspondence of the docking prediction to the experimental result.
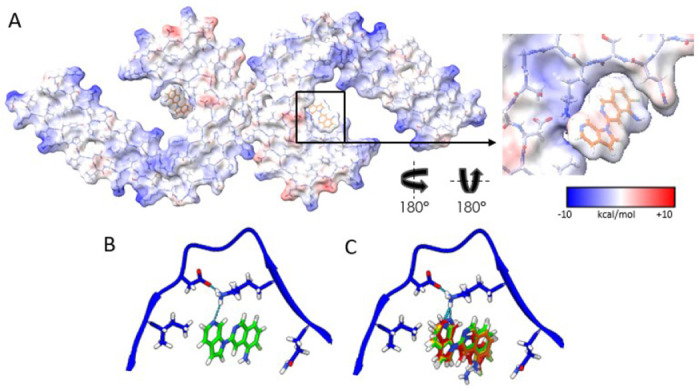
(A) Coulombic energy map depicting the cryo-EM density of the binding pocket, with amino acids from the tau fold and MK-6240. (B) Optimal pose resulting from MK-6240 binding via symmetry docking, revealing hydrogen bonding involving the secondary amine of the azaindole ring and K353. Minimized energetic scores align with the manually docked pose of MK-6240 in the cryo-EM density as shown in (B). (C) The top four poses (Green > Yellow > Orange > Red) generated through symmetry docking, showcasing robust alignment and a predilection for the orientation of the secondary amine of the azaindole ring system to facilitate interaction with K353. Flexibility variations toward the solvent-exposed region are observed among these poses.
