Abstract
Variation in gene copy number can alter gene expression and influence downstream phenotypes; thus copy-number variation provides a route for rapid evolution if the benefits outweigh the cost. We recently showed that genetic background significantly influences how yeast cells respond to gene overexpression, revealing that the fitness costs of copy-number variation can vary substantially with genetic background in a common-garden environment. But the interplay between copy-number variation tolerance and environment remains unexplored on a genomic scale. Here, we measured the tolerance to gene overexpression in four genetically distinct Saccharomyces cerevisiae strains grown under sodium chloride stress. Overexpressed genes that are commonly deleterious during sodium chloride stress recapitulated those commonly deleterious under standard conditions. However, sodium chloride stress uncovered novel differences in strain responses to gene overexpression. West African strain NCYC3290 and North American oak isolate YPS128 are more sensitive to sodium chloride stress than vineyard BC187 and laboratory strain BY4743. Consistently, NCYC3290 and YPS128 showed the greatest sensitivities to overexpression of specific genes. Although most genes were deleterious, hundreds were beneficial when overexpressed—remarkably, most of these effects were strain specific. Few beneficial genes were shared between the sodium chloride-sensitive isolates, implicating mechanistic differences behind their sodium chloride sensitivity. Transcriptomic analysis suggested underlying vulnerabilities and tolerances across strains, and pointed to natural copy-number variation of a sodium export pump that likely contributes to strain-specific responses to overexpression of other genes. Our results reveal extensive strain-by-environment interactions in the response to gene copy-number variation, raising important implications for the accessibility of copy-number variation-dependent evolutionary routes under times of stress.
Keywords: copy-number variation, gxE, evolution, fitness cost
Introduction
Many unicellular organisms like budding yeast Saccharomyces cerevisiae live in environments that fluctuate. Yeast cells can exist in a range of habitats, from fruits and trees to insects and human-associated niches (Liti 2015; Bai et al. 2022). Many of these habitats vary over time and space. Sudden environmental changes occur frequently in nature and can include fluctuations in nutrient availability, temperature, exposure to toxins, and other conditions (Deak 2006). Thus, cells have evolved to deal with changing environments including changes that are stressful. Genetic variation has a substantial influence on stress tolerance and response, due in part to neutral genetic drift but also influenced by adaptive changes. For example, strong selective pressure upon copper exposure, rapid freeze-thaw cycles, dessication, and other conditions are thought to have led to selection for specific genetic backgrounds (Landry et al. 2006; Will et al. 2010; Zarin and Moses 2014; Li et al. 2019). A major focus in evolutionary biology has been to understand modes of evolution and the genetic architecture of differences in environmental tolerance. While single-nucleotide changes can influence phenotype, differences in gene copy number provide a driving force, especially in stressful environments (Zmienko et al. 2014; Berman 2016; Steenwyk and Rokas 2018; Lauer and Gresham 2019; Qidwai 2020). Copy number variation (CNV) can impart an immediate effect on gene expression, which in turn can have an immediate influence on phenotype; if the benefits outweigh the costs, CNVs can become fixed (Wagner 2005, 2011; Tang and Amon 2013; Gerstein and Berman 2015; Lauer and Gresham 2019; Ascencio et al. 2021. Yet, how the cost and benefit of CNV varies with genetic background is only beginning to emerge.
We previously showed that the fitness consequences of gene overexpression (OE), used to model CNV, can vary substantially depending on genetic background. We identified shared and unique responses to each of ∼4,700 yeast genes expressed on a high-copy plasmid in 15 different strains of S. cerevisiae selected from diverse niches and locations from around the globe (Robinson et al. 2021). This library expresses each gene from its native regulatory sequences, along with a unique DNA barcode that can be quantified by sequencing; relative fitness of each gene can be inferred by changes in barcode abundance after competitive growth compared to the starting library. While amplification of >400 genes is commonly deleterious to many strains, the majority of fitness effects were seen in only a subset of strains. This reveals that the fitness effects of gene OE vary widely with host genome, implicating extensive strain-by-CNV interactions. This implies that that different genetic backgrounds will have differential access to evolutionary routes that involve CNV; indeed, strains exposed to extreme selection evolve through different mechanisms, including those that leverage CNV and those that do not (Filteau et al. 2015; Greenblum et al. 2015; Bussotti et al. 2018; Gerstein and Berman 2020; Tung et al. 2021).
A major remaining question is how the environment influences genetic variation in the response to CNV. In nature, cells can experience many different conditions and environments; thus, understanding genotype–environment interactions (GxE) on the consequences of CNV is important (Uddin et al. 2015; Xu et al. 2016; Hujoel et al. 2022). Here, we explored this GxE relationship by examining how the response to gene OE varies across strains grown in a stressful condition. We chose sodium chloride (NaCl) as a stress because of the wealth of molecular information on how yeast cells respond to NaCl stress and how cells regulate the response (Hohmann et al. 2007; de Nadal and Posas 2022). Exposing yeast cells to NaCl causes osmotic stress and ion toxicity, which provoke diverse downstream effects including rapid water efflux, increased Na+ influx and concentration in the cytosol, production of internal osmolytes among other metabolic changes, and mobilization of transcriptomic changes, including activation of the environmental stress response (ESR) (Gasch et al. 2000; Chasman et al. 2014; Yenush 2016; Pascual-Ahuir et al. 2018; Arino et al. 2019). Several signaling pathways are known to respond to NaCl stress, including high osmolarity glycerol (HOG) pathway, nutrient responsive kinase Snf1, and Calcineurin (Cyert 2003; Ye et al. 2008; Shashkova et al. 2015; Petrezselyova et al. 2016; Blomberg 2022; de Nadal and Posas 2022). These pathways can mediate defense strategies such as regulating glycerol accumulation, protecting against protein misfolding, and mediating metabolic changes.
To explore how GxE interactions influence the consequences of gene CNV, we expressed the MoBY 2.0 gene OE library (Ho et al. 2009; Magtanong et al. 2011) in four different yeast strains, each grown for 10 generations in 0.7 M NaCl. While many of the detrimental effects were shared across strains, most of the beneficial OE genes were strain specific. Transcriptomic and genomic analysis revealed several important features of strain-specific responses to NaCl and gene CNV, which may translate to strain-specific evolutionary trajectories during times of stress.
Methods
Strains and growth conditions
Strains used in this study include the laboratory strain BY4743 (MATa/α his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 LYS2/lys2Δ0 met15Δ0/MET15ura3Δ0/ura3Δ0), BC187 (Gerke et al. 2006), NCYC3290 (Liti et al. 2009), and YPS128 (Sniegowski et al. 2002). Strains were grown in rich yeast extract, peptone, dextrose (YPD) medium (10 g/L yeast extract, 20 g/L peptone, 20 g/L dextrose) with G418 (200 mg/L) for plasmid selection in shake flasks at 30°C, with or without 0.7 M NaCl. As described previously (Robinson et al. 2021), strains were transformed with the high-copy MoBY 2.0 library (Ho et al. 2009; Magtanong et al. 2011) to at least 5-fold replication (∼25,000 transformants per strain and library of ∼5,000 unique plasmids). Colonies isolated on plates from the transformation were scraped, pooled, and stored at −80°C.
Fitness measurements
The competition experiments were performed as previously described (Ho et al. 2009). Frozen library-transformed stocks were thawed and placed in 100 ml of liquid YPD with 0.7 M NaCl and G418 (200 mg/L), at a starting OD600 of 0.05. Cultures were transferred to fresh media with or without appropriate supplementation after 5 generations to keep cells in log phase. Cells were harvested after 10 generations and cell pellets were stored at −80°C.
Barcode sequencing and analysis
Plasmids were collected from each culture aliquot using QIAprep spin miniprep kits (Qiagen, Hilden, Germany). The pool of barcodes was amplified as previously described (Bai et al. 2022). Samples were pooled, split, and sequenced across three lanes of an Illumina HiSeq Rapid Run using single-end 100 bp reads. Sequencing data are available in the NIH GEO database under accession number GSE226247.
The data were normalized using library-size normalization as described in Robinson et al. (2021), with one modification: some barcodes repeatedly rose to very high read count in the wild strains. To avoid these genes skewing the library normalization, we calculated total sample read counts excluding genes with >50,000 reads, then divided all read counts (including these highly abundant counts) by that normalization factor. Some highly deleterious genes completely drop out of the population after NaCl outgrowth; for these genes, we imputed missing data similarly to what was described previously (Robinson et al. 2021) as follows: for genes that were well measured at the starting point (>20 normalized read counts in all 3 replicates) but missing after NaCl outgrowth, we added 1 pseudocount at generation 10. After library normalization, we scaled all values by 1,000,000 and rounded to the nearest integer for edgeR analysis (Robinson et al. 2010). The processed and normalized data (available in Supplementary Dataset S1) were used as input to edgeR using a linear model with generation and strain as factors. Genes with a false discovery rate (FDR) < 0.05 were considered significant (Benjamini and Hochberg 1995); the output from edgeR is provided in Supplementary Dataset S2. Relative fitness scores were calculated as the log2 ratio of normalized read counts after vs before outgrowth. Hierarchical clustering (Eisen et al. 1998) was performed using Cluster 3.0 and visualized using Java TreeView (Saldanha 2004). Data for YPD rich media without NaCl were taken from Robinson et al. (2021).
We considered genes with a fitness benefit as those with a significant positive fitness effect (FDR < 0.05); because many genes selected in BY4743 and BC187 strains had very small effect sizes, we also applied a magnitude threshold, requiring a log2 fitness effect of at least 0.8564, which was the smallest beneficial fitness effect of significant genes (FDR < 0.05) in NCYC3290 and YPS128. Three hundred and fourteen genes met these criteria in at least 1 of the 4 strains analyzed (Supplementary Dataset S3). Functional and biophysical enrichments were evaluated using Hypergeometric tests, taking P-value ≤ 10−4 as significant.
Transcriptome profiling and analysis
Yeast strains were grown in biological triplicate in rich YPD medium at 30°C with shaking, for three generations to an optical density at 600 nm (OD600) ∼0.5; all strains were grown in parallel for each replicate, allowing paired downstream analysis. Cells grown in rich medium were shifted to media with 0.7 M NaCl, and samples were collected before and at 30 min and 3 h after the shift. Cells were collected by centrifugation, flash frozen, and maintained at −80°C until RNA extraction. Total RNA was extracted by hot phenol lysis (Gasch 2002), digested with Turbo DNase (Invitrogen) for 30 min at 37°C, and precipitated with 5 M lithium acetate for 30 min at −20°C. rRNA depletion was performed using the Ribo-Zero (Yeast) rRNA Removal Kit (Illumina, San Diego, CA), and libraries were generated according to the TruSeq Stranded Total RNA kit and purified using a Axygen AxyPrep MAG PCR Clean-Up Kit (Axygen). The samples were pooled, re-split, and run across three lanes on an Illumina HiSeq 2500 sequencer, generating single-end 100 bp reads, with ∼7,494,848 reads per sample. Sequencing data are available in the NIH GEO database under accession number GSE226246.
Reads were processed using Trimmomatic version 0.3 (Bolger et al. 2014), and mapped to the S288c reference genome (version R64-1-1) with bwa-mem (version 0.7.12-r1039) (Li and Durbin 2009). Read counts for each gene were calculated by HT-Seq (version 0.6.0) (Putri et al. 2022) and normalized using the TMM method in edgeR (Robinson et al. 2010). We used a linear model in edgeR with strain background as a factor and paired replicates, identifying genes differentially expressed in each strain relative to the average of all strains taking FDR <0.05 as significant. Hierarchical clustering (Eisen et al. 1998) was performed by Cluster 3.0 and visualized using Java TreeView (Saldanha 2004). Functional analysis and enriched GO categories for each sample were obtained using hypergeometric tests, taking P-value ≤ 10−4 as significant. EdgeR identified 1,114 genes whose fold change in expression after NaCl was different in at least one strain compared to the mean fold change across strains (FDR < 0.05, Supplementary Dataset S4, Table 1). Relative expression was represented as the log2 (fold change) in each strain, comparing normalized read counts 30 min or 3 h after vs before NaCl treatment in each strain. Comparing basal (unstressed) expression across strains identified 608 genes whose expression was significantly different from the mean (FDR < 0.05) in at least one of the strains (Supplementary Dataset S4, Table 2).
ENA gene CNV analysis
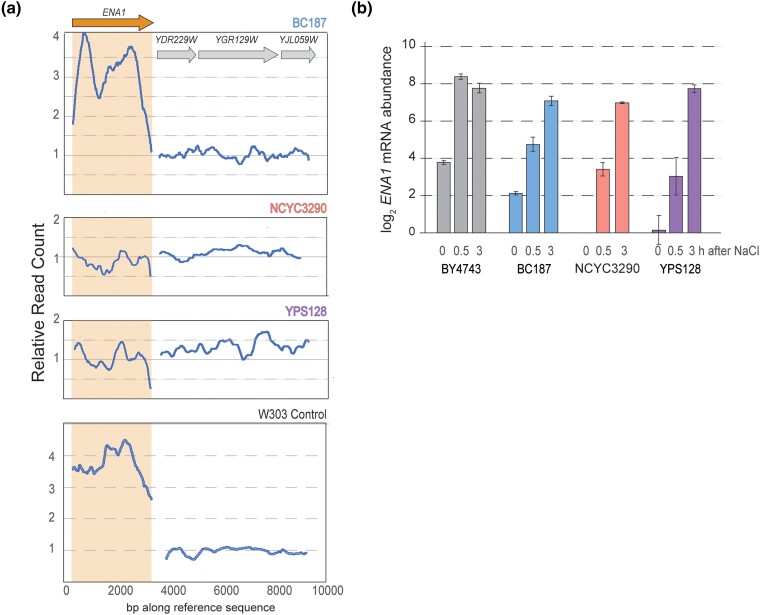
We analyzed previously published DNA-seq data generated for these strains and mapped to the S288c reference sequence (Liti et al. 2009; Hose et al. 2015; Sardi et al. 2018). We normalized read counts at all positions to the genomic media read count and then randomly selected three representative genes from three different chromosomes (YDR229W, YGR125W, YJL059W) whose DNA-seq read abundance was at the median read depth of the genome. These genes were taken to represent genes at single copy per haploid genome. To avoid mapping errors to the S288c reference that contains multiple highly similar ENA genes, we re-mapped reads to a reference sequence consisting of S288c ENA1 (YDR040C) and the three representative single-copy genes using bwa-mem (Li and Durbin 2009) and then used samtools mpileup function (Li et al. 2009) to plot the coverage of each base pair in the reference sequence. Read counts were normalized to the median coverage of the three reference genes. Figure 5 shows the running average of normalized read count over 500 bp windows.
Fig. 5.
Strains vary in ENA copy number and expression. a) Relative DNA-seq read count across a representative ENA gene (left trace, orange arrow) and three representative single-gene copies (right trace, gray arrows) in each of the three wild strains (see Methods). The plot shows the running average across 500 bp windows from left to right along each sequence. As a control, sequence is shown for a related laboratory strain W303, sequenced with the same pipeline as the wild strains and known to carry four ENA copies. b) Average and standard deviation (n = 3) of log2 fold change in ENA1 mRNA abundance before and after NaCl treatment, normalized to NCYC3290 basal levels at time 0 min.
Results
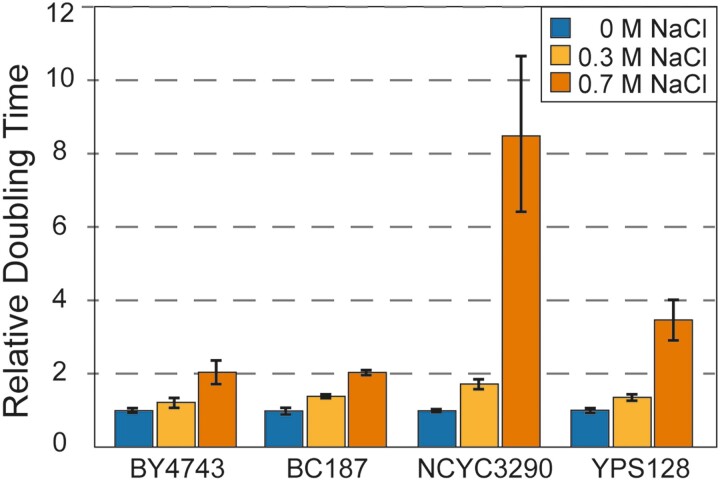
To investigate the effects of gene OE under NaCl stress, we focused on a subset of diploid strains analyzed in a previous study from our lab, selecting three strains representing distinct genetic lineages: West African strain NCYC3290, vineyard strain BC187, and North American oak isolate YPS128, along with common lab strain BY4743 as a well-studied reference (Liti et al. 2009; Peter et al. 2018). In addition to genetic differences, these strains display extensive phenotypic diversity in several different environmental conditions, including osmotic stress (Kvitek et al. 2008; Warringer et al. 2011; Peter et al. 2018; Sardi et al. 2018). We first measured strain growth rates in 0.3 and 0.7 M NaCl to characterize NaCl tolerance. Growth of all strains was reduced in NaCl compared to rich medium (Fig. 1. However, there was significant variation in strain responses. West African NCYC3290 and to a lesser extent oak-soil YPS128 strains were much more sensitive to the higher dose of NaCl, indicated by their 8.5× and 3.5× reduction in doubling time compared to only ∼2× reduction in vineyard strain BC187 and lab strain BY4743. We chose the higher dose of NaCl to interrogate how this environment influences strain-specific responses to CNV.
Fig. 1.
Strains vary in salt sensitivity. Average and standard deviation (n = 3) of doubling times of each strain grown in the absence or presence of NaCl according to the key, normalized to the unstressed growth rate of that strain.
Next, we quantified the fitness effects of gene OE across different genetic backgrounds when cells were subjected to a stressful environment of 0.7 M NaCl. Each strain was transformed with the library, which includes ∼5,000 genes cloned from S288c along with their native upstream and downstream sequences, cloned onto a 2-micron replication plasmid (Ho et al. 2009; Magtanong et al. 2011). Each plasmid also carries a DNA barcode, which can be identified and quantified through deep sequencing of the pooled library. We note that one limitation of this approach is that some genes that are not expressed in salt conditions will not show increased expression and thus will be scored as neutral. Cells were inoculated into rich medium and an aliquot collected before (‘0 generation” sample) and after 10 generations of growth in 0.7 M NaCl, in biological triplicate. Gene abundances before and after outgrowth were identified by deep sequencing of plasmid barcodes.
Past analysis showed that these three wild strains carry 2–3 copies of the 2-micron plasmid from this library, per haploid genome, making results from these strains directly comparable. The laboratory strain is distinct from many wild strains and carries ∼11 copies per haploid genome (Robinson et al. 2021). To account for these differences, we normalized sequencing data for each library and calculated the log2 ratio of normalized barcode read counts after NaCl treatment vs before (see Methods). This reflects the relative fitness cost of each gene compared to the library expressed in that strain. Plasmids that carry genes that are detrimental when OE drop in frequency in the population, either because of reduced cell growth in the population or because cells suppress the abundance of toxic plasmids (Makanae et al. 2013), both of which we interpret as a relative fitness defect. In contrast, beneficial plasmids will rise in frequency in the population over time. We used linear modeling to identify genes with a significant fitness effect in each strain (FDR < 0.05).
Common and unique fitness consequences across strains and environments
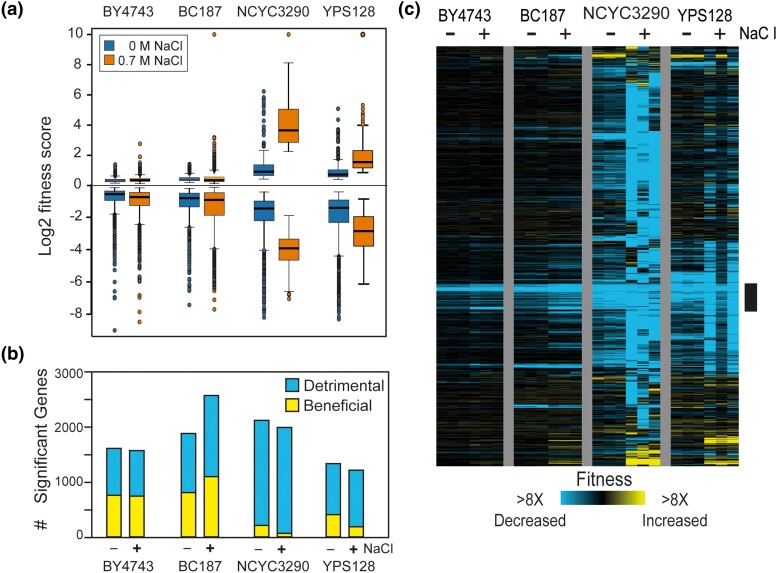
We identified a total of 3,644 genes (from 4,133 interrogated) whose OE produced a relative fitness effect in at least one strain growing under NaCl stress (FDR < 0.05, Fig. 2). Yet the number and magnitude of fitness costs varied substantially. Lab strain BY4743 showed the least impact of gene OE, both in terms of number of deleterious OE genes and their impact on fitness (Fig. 2a and b), followed by BC187 which also showed relatively mild fitness effects for most genes. For both of these strains, the distribution of relative fitness costs was similar with and without NaCl, consistent with their ability to grow well in salt-containing medium (Fig. 1). In contrast, the NaCl-sensitive strains showed several key differences. First, both strains were more sensitive to gene OE in the absence of stress, indicated both by the number of deleterious OE genes and their greater effect sizes compared to NaCl-tolerant strains. Second, both strains—but especially the most NaCl sensitive, NCYC3290—were much more sensitive to gene OE in the presence of NaCl. For example, the vast majority of OE genes were deleterious in NCYC3290 and with severe fitness costs (Fig. 2a–c).
Fig. 2.
Fitness consequences vary by strain. a) Distribution of average log2 fitness scores in each strain grown 10 generations in rich medium in the absence of NaCl (blue) or in medium with 0.7 M NaCl (orange). b) The number of detrimental (blue) or beneficial (yellow) genes in each strain and media condition. c) Hierarchical clustering of 3,644 genes with a fitness effect (FDR < 0.05) in at least one strain grown in NaCl stress. Each row is a specific gene, each column is a biological replicate of that strain grown in the absence (−) or presence (+) of NaCl. Blue and yellow values represent genes that dropped or rose in abundance during competitive growth, reflecting decreased or increased fitness effects according to the key. The black bar indicates a cluster of genes whose OE is detrimental to varying degrees in all strains and under both conditions. Data from NaCl-free conditions were taken from Robinson et al. (2021).
To explore the patterns of fitness costs across strains and conditions, we hierarchically clustered OE genes that had a relative fitness effect in at least one strain. The resulting heat map illustrates the commonalities and differences across strains and conditions. We identified one cluster of ∼200 OE genes (Fig. 2c, black bar) that were deleterious in all four 4 strains in both YPD and NaCl stress conditions, to varying degrees. Consistent with past work from our lab (Robinson et al. 2021), this gene group was heavily enriched for genes involved in translation, including ribosomes and ribosome biogenesis factors, protein folding factors, and genes repressed during stress in the environmental stress response (ESR, P < 10−4, Hypergeometric tests). But even for these commonly deleterious OE genes, the magnitude of the effect varied, especially for the wild strains where the fitness cost was more severe in the presence of NaCl. These results are consistent with the notion that the cost of gene OE is greater in strains already experiencing suboptimal conditions (see Discussion).
Beneficial genes vary widely across strains
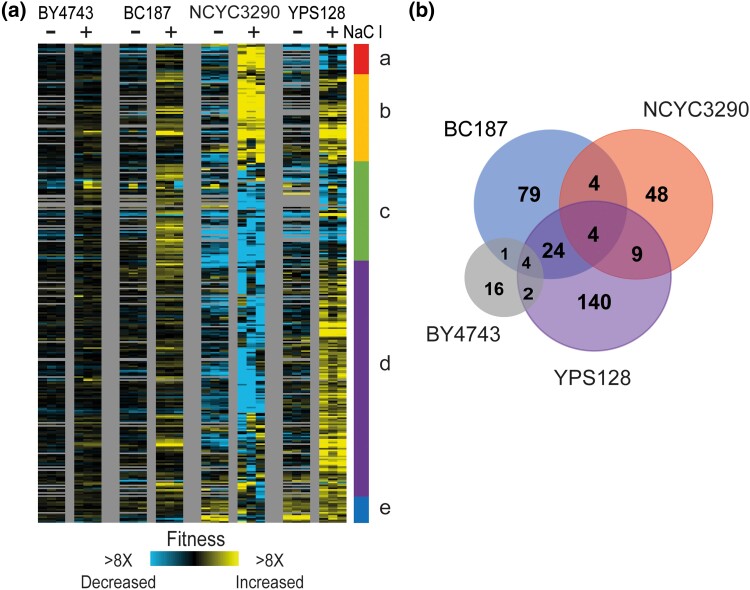
We were particularly interested in genes whose OE provides a benefit under NaCl stress, since these may provide adaptive value. We identified and hierarchically clustered 331 genes whose OE produced a significant benefit in NaCl over an effect threshold in at least one strain (Fig. 3a, see Methods). BY4743 and BC187 had a substantial number of genes that passed our statistical threshold but were of very small effect size (hence the use of a magnitude threshold, identifying only 23 beneficial OE genes for BY4743). In contrast, 116, 65, and 183 genes met our criteria of providing a benefit to BC187, NCYC3290, and YPS128, respectively. Interestingly, there was only small overlap in which genes were beneficial during NaCl stress, even for the two NaCl-sensitive strains (Fig. 3b). This strongly implies that the genetic basis for NaCl sensitivity is different in the two sensitive strains.
Fig. 3.
Beneficial genes vary widely by strain. a) Hierarchical clustering of 314 genes that were beneficial in at least one strain (see Methods), as described in Fig. 2; gray indicates missing values. Several clusters were enriched for functional groups (P < 1 × 10−4, hypergeometric test) including genes involved in a) cell-cycle entry, c) negative regulation of heterochromatin, d) cytoplasmic mRNA processing body assembly, snoRNA processing. b) Overlap of beneficial genes identified in each strain.
Only four genes were scored as beneficial to all three wild strains but not BY4743 (Fig. 3b). These included calmodulin kinase CMK2, Snf1-related kinase HAL5 that regulates ion tolerance (Mulet et al. 1999; Tumolo et al. 2020), CK2-kinase subunit CKA1 that has been implicated in NaCl stress (Bidwai et al. 1995; Kanhonou et al. 2001), and GCD10 that encodes a tRNA methyl transferase. It is interesting that three of the four genes are kinases that could have a wide range of downstream effects on physiology. Expanding to genes shared between the two sensitive strains identified several other genes involved in cation homeostasis including sodium and other-cation transporter QDR2, kinase SAT4 involved in sodium tolerance (Mulet et al. 1999), and diacylglycerol kinase DGK1 (itself a target of CK2 (Qiu et al. 2016)). This group was also enriched for genes regulated by the HOG-regulated osmotic stress transcription factor Sko1 (P = 5 × 10−4, Hypergeometric test), which is interesting in the context of the NaCl sensitivity of these strains. Interestingly, the total set of genes whose OE was beneficial to YPS128 were enriched for genes involved in mRNA P-body and stress granule assembly and ergosterol biosynthesis (P < 1 × 10−4, Hypergeometric test). BC187 also benefited from OE of many RNA binding proteins, several of which were shared with YPS128. In contrast, genes beneficial to NCYC3290 were enriched for those involved in glycosyl-group transferase activity and flocculation. Together, these results indicate that the fitness consequences of beneficial genes are largely strain specific, in some cases causing opposing fitness effects in different strains (Fig. 3, clusters a, c, and d).
Transcriptomic analysis implicates genetic modifiers
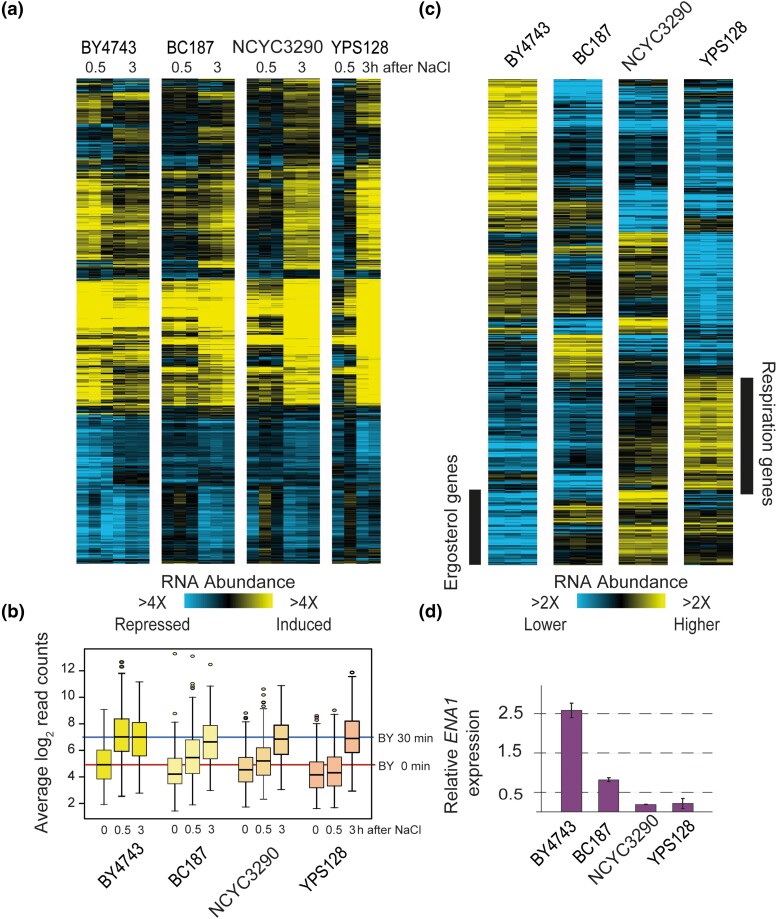
The low overlap in beneficial OE genes among the NaCl-sensitive strains suggests that NaCl sensitivity is explained by different genetic or physiological limitations. In attempt to better understand background-specific effects that could influence these differences, we characterized each strain's transcriptomic response to NaCl shock. Cells grown in rich medium were shifted to media with 0.7 M NaCl, and samples were collected before and at 30 min and 3 h after the shift, in biological triplicate (see Methods). We chose these timepoints to explore the response to NaCl immediately after acute shock and at a later timepoint that represents the acclimated state. We expected that the sensitive strains may show substantial differences in transcriptomic response to the shock, reflecting their increased NaCl sensitivity; however this was not the case. Using a linear model to identify strain-by-environment interactions, we identified 1,141 genes whose expression to NaCl differed across strains at one or both time points after shock (FDR < 0.05, see Methods). The four strains showed fairly similar gene expression changes at both induced and repressed genes (Fig. 4a)—there were no large gene groups that were uniquely induced or repressed in any of the strains. Furthermore, the magnitude of many of those gene expression changes was similar across strains. This was especially surprising for the sensitive strains, since we expected that they may exhibit larger magnitude changes if they experience a stronger stress from 0.7 M NaCl treatment.
Fig. 4.
Gene expression differences implicate strain-specific physiology. a) Hierarchical clustering of 1,141 genes whose log2(fold change) in response to NaCl was different from the mean in at least one of the four strains (FDR < 0.05). As shown for Fig. 2, except that here values represent relative mRNA abundance in stressed samples vs the prestressed sample from that strain. Each column represents one of three biological replicates (or two in the case of YPS128). b) The distribution of log2 normalized read counts for genes induced in the ESR across strains and time points. The median abundance of genes in the laboratory strain before and at 30 min after NaCl treatment is indicated with red and blue lines. c) Hierarchical clustering of 608 genes whose basal expression before NaCl is different from the mean in at least one of the four strains (FDR < 0.05). Each column is one of three biological replicates of cells growing in rich medium in the absence of stress, where log2 values represent expression relative to the mean of all strains according to the key. d) Average and standard deviation of ENA1 expression in the absence of stress relative to the mean of all strains.
Despite these global similarities, on closer inspection we noticed a difference in the timing of the response, especially compared to the BY4743 laboratory strain. The lab strain had much larger changes in expression at 30 min after NaCl shock than the other strains, and many of these expression differences were already subsiding by 3 h. Genes induced in the ESR provide a good representation (Fig. 4b): these genes show much larger and earlier expression changes in the laboratory strain. In contrast, all three wild strains showed delayed expression changes, of genes induced in the ESR (Fig. 4b) and other genes more broadly (Fig. 4a). Much of the transcriptome response is regulated by the Hog1 kinase that responds to osmotic stress. Interestingly, it is well known that the timing of Hog1 signaling varies with the dose of ionic stress: at higher doses of stress, signaling is delayed and produces a delayed transcriptomic response (Maeda et al. 1995; O'Rourke and Herskowitz 2004; Hersen et al. 2008; Granados et al. 2017). This suggested that wild strains could all be experiencing a higher level of NaCl stress that delays the transcriptomic response (yet does not grossly change the magnitude or genes in the response, see Discussion). There was little relationship between genes expression and gene fitness benefits in each strain (aside of YPS128, in which seven beneficial genes were more highly induced in that strain upon NaCl treatment). In fact, only 20% of genes beneficial in one or more strain (Fig. 3b) were differentially expressed in any strain (Fig. 4a), and nearly half of those (43%) were repressed by NaCl across strains. This is not entirely surprising, since most genes with expression changes during NaCl treatment have no bearing on surviving NaCl treatment, and many genes important for NaCl survival are actually repressed at the transcript level (Giaever et al. 2002; Berry and Gasch 2008; Berry et al. 2011).
Although the transcript changes to NaCl were not wildly different across strains aside of the timing, we wondered if basal expression differences in the strains, before NaCl exposure, could be informative. We therefore identified 608 genes whose expression was significantly different in at least one strain compared to the mean (FDR < 0.05, see Methods). Here, expression differences were more noticeable across the strains (Fig. 4c). In fact, the laboratory strain was a clear outlier compared to the three wild strains: one large group of genes was expressed significantly higher in BY4743 in the absence of stress, and this group was enriched for stress-defense genes (see also Fig. 4b), oxidoreductases, amino acid biosynthesis genes, transporters, and genes encoding proteins localized to the membrane and vacuole (P < 1e−4, Hypergeometric test). This result suggests that BY4743 is already prepared for stress even before exposure (see Discussion). A second group of genes was expressed significantly lower in the lab strain, and these were heavily enriched for genes encoding respiration factors and ergosterol biosynthesis genes and targets of Hap1 (Fig. 4c). Some of these differences may result from known polymorphisms in S288c-derived strains that affect the regulation of those genes (including MKT1, MIP1, HAP1 along with auxotrophic markers that affect mitochondrial and respiratory functions and/or ergosterol-gene expression (Gaisne et al. 1999; Young and Court 2008; Dimitrov et al. 2009)).
Remarkably, among the genes with much higher basal expression in BY4743 were several linked directly to Na+ transport, including ENA1, ENA5, and NHA1. In fact, ENA genes coding for P-type ATPase sodium pumps are known to have undergone tandem duplications in different strains including BY4743, which harbors three copies per haploid genome, each with high sequence homology (Haro et al. 1991; Wieland et al. 1995; Doniger et al. 2008; Strope et al. 2015; Barbitoff et al. 2021). ENA genes are important components in the saline detoxification (although they are not present in the MoBY 2.0 library), and strains with higher ENA copy number are well known to have correspondingly higher sodium tolerance (Daran-Lapujade et al. 2009; Warringer et al. 2011; Wilkening et al. 2014; Sirr et al. 2018; Pontes et al. 2019). We plotted the relative abundance of RNA-seq reads mapping to ENA1 as a representative and found that expression varied wildly across strains. The two sensitive strains showed very low expression of ENA1 relative to the other strains; expression in vineyard strain BC187 was ∼4× higher than NCYC3290, while expression in lab strain BY4743 was 14× higher (Fig. 4c). These differences raised the possibility of underlying gene copy-number differences.
To investigate if underlying expression differences were due to natural CNV of these genes, we interrogated DNA-seq reads mapped to a single ENA gene copy relative to three representative genes present in single copy per haploid genome (see Methods). NCYC3290 and YPS128 both showed per-base coverage that was similar to the single copy genes, barring some fluctuation in read depth that may be due to polymorphism-dependent mapping errors: the median read count for ENA1 vs the median of the single-copy genes was 1.1 and 1.0 for NCYC3290 and YPS128, respectively (Fig. 5a). In contrast, BC187 showed read distribution that was ∼3–4× higher across most of the gene, excluding the very amino terminus (median ENA1 read count vs single-copy genes of 3.1). As a control for our analysis method, we plotted reads for a related lab strain, W303 that was sequenced with the same pipeline as the wild strains—the median read depth of ENA1 vs single-copy genes was 3.7, consistent with the known four ENA copies in this strain (Colombi et al. 2023). The reduced coverage at the ends of the gene could be due to incomplete gene duplication, or it may reflect substantial polymorphisms between BC187 and the reference genome that obscure copy number. The latter possibility is supported by the known high rate of evolution of ENA genes, including at least one case of introgression from S. paradoxus (Doniger et al. 2008; Daran-Lapujade et al. 2009; Warringer et al. 2011; Sirr et al. 2018). We conclude that BC187 has 3 ENA gene copies per haploid genome, although the functionality of all copies remains to be explored.
Strains with higher abundance of ENA1 and related sodium pumps are well known to have increased tolerance to sodium stress (Ferrando et al. 1995; Tenney and Glover 1999; Crespo et al. 2001; Daran-Lapujade et al. 2009; Warringer et al. 2011; Pontes et al. 2019). Interestingly, however, strain-specific differences in ENA transcript abundance were not fully explained by differences in ENA copy number: although BY4743 and BC187 both harbor 3 ENA copies per haploid genome, basal expression in the lab strain was 3-fold higher than BC187 (Fig. 4c); likewise, basal expression in BC187 was >4-fold higher than the sensitive strains. These results suggested that the response to NaCl could be influenced both by variation in ENA gene copy number and by ENA gene regulation. ENA genes are also transcriptionally induced by NaCl (Proft and Serrano 1999; Tenney and Glover 1999; Ruiz et al. 2003), and in fact several studies have observed natural variation in ENA transcriptional regulation (McDaniel et al. 2018; Sirr et al. 2018). We plotted ENA1 mRNA abundance before and after NaCl in each strain, normalized to NCYC3290 basal levels. While BY4743 harbored the highest ENA1 expression in the absence of stress, the strain further induced ENA1 another 25-fold within 30 minutes after 0.7 M NaCl exposure. Remarkably, the other strains all induced ENA1 expression to within 2-fold of BY4743 mRNA levels, albeit with delayed kinetics. While induction of ENA1 may help with the acclimation to continued salt stress (McDaniel et al. 2018), it is likely that the basal expression levels influence the immediate survival after rapid-onset salt stress. Thus, we propose that although strains retain the ability to induce ENA1 expression after stress, the low starting mRNA levels likely contribute to variations in the ability to survive the initial NaCl exposure—this may also explain differences in the fitness cost of other OE genes, including OE of other sodium transporters and ion-response regulators whose duplication provides a major benefit to wild strains but has no effect in BY4743 (see Discussion).
Discussion
Our results show that environmental stress has a major influence on the fitness consequences of gene OE, and those effects vary substantially across genetically distinct individuals. That environmental stress influences the cost of gene OE may not seem surprising from a physiological point of view, and this is consistent with longstanding models on the effects of stress on mutational tolerance (Szafraniec et al. 2001; Fry and Heinsohn 2002; Elena and de Visser 2003; Kishony and Leibler 2003). However, our results have major implications for how strain and environment affect the fitness costs of gene CNV, which is an important route to rapid evolution. These trends are almost certainly true for other stresses beyond NaCl treatment studied here. We previously showed only small overlap in OE fitness consequences to toxin tolerance in wild strains growing in industrial conditions (Sardi et al. 2016). We subsequently showed that the mechanisms of tolerating those conditions vary substantially across strains, suggesting that different genes will be beneficial depending on the physiological weaknesses of each individual (Sardi et al. 2018). We propose that Strain-by-Environment-by-CNV interactions are prominent and could produce substantial variation in the evolutionary trajectories accessible to different individuals and over space and time.
Simply duplicating a gene's copy number can increase its expression, at least for genes that are not dosage regulated (Stranger et al. 2007; Hose et al. 2015; Cromie et al. 2017; Ascencio et al. 2021). Amplification of many genes is deleterious even in the absence of stress, likely due to the increased burden of producing extra DNA, RNA, and protein but also due to internal imbalances caused therein (Wagner 2011; Birchler and Veitia 2021). NaCl treatment exacerbated the deleterious effects of many of those genes, as might be expected (Szafraniec et al. 2001; Fry and Heinsohn 2002; Elena and de Visser 2003; Kishony and Leibler 2003). But even for the same concentration of NaCl, strains more sensitive to NaCl showed both more deleterious responses and larger effect sizes during NaCl and compared to more tolerant strains (Fig. 2). This is consistent with the idea that the cost of CNV is generally worse when cells are already taxed, in accordance with previous implications from our work (Robinson et al. 2021). In many cases, the increased cost of gene OE may be independent of the gene's function (Wagner 2005, 2011; Robinson et al. 2021) and could simply represent compounded burdens of producing extra protein during an energy-consuming stress response. In other cases, specific gene functions may be counterproductive during the NaCl acclimation and thus uniquely deleterious in that environment. For example, increased expression of functional aquaporin water transporters is detrimental during osmotic shock, due to passive water loss that exacerbates stress-induced water efflux (Will et al. 2010). Although increased aquaporin expression is beneficial in other environments, the cost of that increase is harder to overcome in high-osmolar conditions. The implication is that different evolutionary routes will be more or less accessible depending on the environmental context.
Adaptive benefits of CNVs are well known during environmental stress (Zmienko et al. 2014; Berman 2016; Steenwyk and Rokas 2018; Lauer and Gresham 2019; Qidwai 2020), and thus we expected to find some genes whose OE is uniquely beneficial during NaCl exposure compared to standard conditions. The surprise was that there was little overlap in beneficial genes across strains, including the more sensitive strains. Part of the low overlap results from inclusion of the laboratory strain, BY4743. This strain showed the fewest beneficial genes, and most of those genes produced only mild benefits. This along with growth responses shown in Fig. 1 is consistent with the notion that BY4743 is fairly tolerant of NaCl, and thus there is little room for improvement. Our genomic analyses raised several possible explanations. Unstressed BY4743 shows higher expression of many genes directly related to stress defense (Fig. 4b and c), including genes linked to osmotic and other stress defenses. Furthermore, BY4743 displays very high expression of ENA genes encoding sodium efflux pumps even in the absence of stress, likely due to both natural ENA gene amplification and higher expression from one or more copies. Together, these results strongly suggest that BY4743 is already prepared for NaCl stress before exposure, requiring less acclimation effort upon treatment. This preparedness could also explain the accelerated transcriptomic response to NaCl shock (Fig. 4a and b): higher basal stress tolerance coupled with immediate Na+ efflux could result in a lower effective dose of NaCl, which is known to produce a faster signaling response (Maeda et al. 1995; O'Rourke and Herskowitz 2004; Hersen et al. 2008; Granados et al. 2017). Whether these expression differences have been selected due to laboratory domestication or merely accumulated due to neutral drift, these results are consistent with the idea that there is little adaptive benefit for most OE genes in the lab strain under these conditions.
The results in the lab strain are perhaps not surprising, since laboratory strains are often outliers in their responses (Hose et al. 2015, 2020; Gallone et al. 2016; Gasch et al. 2016)—but, we were surprised to see the low overlap in beneficial OE genes across wild strains, especially NaCl-sensitive NCYC3290 and YPS128 (Fig. 3b). The beneficial gene sets for each strain were enriched for distinct functions; the exception was shared enrichment of osmo-responsive Sko1 targets among the beneficial genes shared by the two sensitive strains. Yet, there were several genes common to two or all three of the wild strains (albeit with different effect sizes, often greatest in the sensitive strains, Supplementary Dataset S2). These included genes linked to cation stress, including kinases Hal5 and Sat4 and transporter Qdr1 that are known to modulate ion tolerance (Mulet et al. 1999; Vargas et al. 2007; Rios et al. 2013). It is interesting that these genes had virtually no benefit in the lab strain that is already well equipped for Na+ stress.
We propose that the significant fitness benefit of these genes to wild strains but not BY4743 could be impacted by variation in abundance of ENA ATPase Na+ pumps. The sensitive strains harbor only one copy of ENA per haploid genome, whereas BC187 carries 3 copies that remain lower expressed than in BY4743 (Fig. 5). Given the importance of ENA expression in NaCl tolerance (Daran-Lapujade et al. 2009; Pontes et al. 2019), we propose that differences in ENA copy number and expression influence which other OE genes will be beneficial. This would explain why strains with lower ENA mRNA abundance greatly benefit from OE of other genes directly involved in sodium efflux, whereas BY4743 receives no benefit from OE of those genes. Interestingly, several other genes uniquely beneficial to one or more wild strains transcriptionally up-regulate ENA1, including CK2 whose OE benefited all strains and CRZ1 that was highly beneficial to BC187 (Tenney and Glover 1999; Mendizabal et al. 2001; Petrezselyova et al. 2016). These possibilities highlight the potential for genetic interactions between CNVs, since the impact of one gene's amplification is dependent on another gene's copy number.
In all, this study adds to the body of evidence that the impact of a mutation, in this case gene OE to mimic the effects of CNV, depends on not only the genomic context but also environment. Ultimately, the combined effect of genomic and environmental variation can perhaps best be considered as Gene-by-System interactions (Sardi and Gasch 2018), where the impact of a gene's CNV depends on the physiological state of the cell. A remaining challenge is developing statistical models that can both represent Gene-by-System interactions and uncover the biology behind them.
Supplementary Material
Acknowledgments
We thank members of the Gasch Lab for helpful discussions.
Contributor Information
DeElegant Robinson, Center for Genomic Science Innovation, University of Wisconsin-Madison, Madison, WI 53704, USA.
Elena Vanacloig-Pedros, Center for Genomic Science Innovation, University of Wisconsin-Madison, Madison, WI 53704, USA; Great Lakes Bioenergy Research Center, University of Wisconsin-Madison, Madison, WI 53704, USA.
Ruoyi Cai, Center for Genomic Science Innovation, University of Wisconsin-Madison, Madison, WI 53704, USA.
Michael Place, Center for Genomic Science Innovation, University of Wisconsin-Madison, Madison, WI 53704, USA; Great Lakes Bioenergy Research Center, University of Wisconsin-Madison, Madison, WI 53704, USA.
James Hose, Center for Genomic Science Innovation, University of Wisconsin-Madison, Madison, WI 53704, USA.
Audrey P Gasch, Center for Genomic Science Innovation, University of Wisconsin-Madison, Madison, WI 53704, USA; Great Lakes Bioenergy Research Center, University of Wisconsin-Madison, Madison, WI 53704, USA; Department of Medical Genetics, University of Wisconsin-Madison, Madison, WI 53704, USA.
Data availability
Barcode sequencing data are available in the NIH GEO database under accession number GSE226247. RNA sequencing data are available in the NIH GEO database under accession number GSE226246.
Supplemental material available at G3 online.
Funding
This work was funded by R01 GM147271 to APG and in part by grant DE-S0018409 from the United States Department of Energy.
Literature cited
- Arino J, Ramos J, Sychrova H. Monovalent cation transporters at the plasma membrane in yeasts. Yeast. 2019;36(4):177–193. doi: 10.1002/yea.3355. [DOI] [PubMed] [Google Scholar]
- Ascencio D, Diss G, Gagnon-Arsenault I, Dube AK, DeLuna A, DeLuna A, Landry CR. Expression attenuation as a mechanism of robustness against gene duplication. Proc Natl Acad Sci U S A. 2021;118(6):e2014345118. doi: 10.1073/pnas.2014345118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai FY, Han DY, Duan SF, Wang QM. The ecology and evolution of the Baker's yeast Saccharomyces cerevisiae. Genes (Basel). 2022;13(2):230. doi: 10.3390/genes13020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbitoff YA, Matveenko AG, Matiiv AB, Maksiutenko EM, Moskalenko SE, Drozdova PB, Polev DE, Beliavskaia AY, Danilov LG, Predeus AV, et al. . Chromosome-level genome assembly and structural variant analysis of two laboratory yeast strains from the Peterhof Genetic Collection lineage. G3 (Bethesda). 2021;11(4):jkab029. doi: 10.1093/g3journal/jkab029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Berman J. Ploidy plasticity: a rapid and reversible strategy for adaptation to stress. FEMS Yeast Res. 2016;16(3):fow020. doi: 10.1093/femsyr/fow020. [DOI] [PubMed] [Google Scholar]
- Berry DB, Gasch AP. Stress-activated genomic expression changes serve a preparative role for impending stress in yeast. Mol Biol Cell. 2008;19(11):4580–4587. doi: 10.1091/mbc.e07-07-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DB, Guan Q, Hose J, Haroon S, Gebbia M, Heisler LE, Nislow C, Giaever G, Gasch AP. Multiple means to the same end: the genetic basis of acquired stress resistance in yeast. PLoS Genet. 2011;7(11):e1002353. doi: 10.1371/journal.pgen.1002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidwai AP, Reed JC, Glover CV. Cloning and disruption of CKB1, the gene encoding the 38-kDa beta subunit of Saccharomyces cerevisiae casein kinase II (CKII). Deletion of CKII regulatory subunits elicits a salt-sensitive phenotype. J Biol Chem. 1995;270(18):10395–10404. doi: 10.1074/jbc.270.18.10395. [DOI] [PubMed] [Google Scholar]
- Birchler JA, Veitia RA. One hundred years of gene balance: how stoichiometric issues affect gene expression, genome evolution, and quantitative traits. Cytogenet Genome Res. 2021;161(10–11):529–550. doi: 10.1159/000519592. [DOI] [PubMed] [Google Scholar]
- Blomberg A. Yeast osmoregulation—glycerol still in pole position. FEMS Yeast Res. 2022;22(1):foac035. doi: 10.1093/femsyr/foac035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussotti G, Gouzelou E, Cortes Boite M, Kherachi I, Harrat Z, Eddaikra N, Mottram JC, Antoniou M, Christodoulou V, Bali A, et al. . Leishmania genome dynamics during environmental adaptation reveal strain-specific differences in gene copy number variation, karyotype instability, and telomeric amplification. mBio. 2018;9(6):e01399-18. doi: 10.1128/mBio.01399-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasman D, Ho YH, Berry DB, Nemec CM, MacGilvray ME, Hose J, Merrill AE, Lee MV, Will JL, Coon JJ, et al. . Pathway connectivity and signaling coordination in the yeast stress-activated signaling network. Mol Syst Biol. 2014;10(11):759. doi: 10.15252/msb.20145120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombi P, King DE, Williams JF, Lusk CP, King MC. LEM domain proteins control the efficiency of adaptation through copy number variation. bioRxiv. 2023.preprint: not peer reviewed.
- Crespo JL, Daicho K, Ushimaru T, Hall MN. The GATA transcription factors GLN3 and GAT1 link TOR to salt stress in Saccharomyces cerevisiae. J Biol Chem. 2001;276(37):34441–34444. doi: 10.1074/jbc.M103601200. [DOI] [PubMed] [Google Scholar]
- Cromie GA, Tan Z, Hays M, Jeffery EW, Dudley AM. Dissecting gene expression changes accompanying a ploidy-based phenotypic switch. G3 (Bethesda). 2017;7(1):233–246. doi: 10.1534/g3.116.036160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert MS. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem Biophys Res Commun. 2003;311(4):1143–1150. doi: 10.1016/S0006-291X(03)01552-3. [DOI] [PubMed] [Google Scholar]
- Daran-Lapujade P, Daran JM, Luttik MA, Almering MJ, Pronk JT, Kötter P. An atypical PMR2 locus is responsible for hypersensitivity to sodium and lithium cations in the laboratory strain Saccharomyces cerevisiae CEN.PK113-7D. FEMS Yeast Res. 2009;9(5):789–792. doi: 10.1111/j.1567-1364.2009.00530.x. [DOI] [PubMed] [Google Scholar]
- Deak T. Environmental factors influencing yeasts. In: Biodiversity and Ecophysiology of Yeasts. Heidelberg: Springer; 2006. p. 155–174. [Google Scholar]
- de Nadal E, Posas F. The HOG pathway and the regulation of osmoadaptive responses in yeast. FEMS Yeast Res. 2022;22(1):foac013. doi: 10.1093/femsyr/foac013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov LN, Brem RB, Kruglyak L, Gottschling DE. Polymorphisms in multiple genes contribute to the spontaneous mitochondrial genome instability of Saccharomyces cerevisiae S288C strains. Genetics. 2009;183(1):365–383. doi: 10.1534/genetics.109.104497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger SW, Kim HS, Swain D, Corcuera D, Williams M, Yang S-P, Fay JC. A catalog of neutral and deleterious polymorphism in yeast. PLoS Genet. 2008;4(8):e1000183. doi: 10.1371/journal.pgen.1000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95(25):14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elena SF, de Visser JA. Environmental stress and the effects of mutation. J Biol. 2003;2(2):12. doi: 10.1186/1475-4924-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrando A, Kron SJ, Rios G, Fink GR, Serrano R. Regulation of cation transport in Saccharomyces cerevisiae by the salt tolerance gene HAL3. Mol Cell Biol. 1995;15(10):5470–5481. doi: 10.1128/MCB.15.10.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filteau M, Hamel V, Pouliot MC, Gagnon-Arsenault I, Dube AK, Landry CR. Evolutionary rescue by compensatory mutations is constrained by genomic and environmental backgrounds. Mol Syst Biol. 2015;11(10):832. doi: 10.15252/msb.20156444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry JD, Heinsohn SL. Environment dependence of mutational parameters for viability in Drosophila melanogaster. Genetics. 2002;161(3):1155–1167. doi: 10.1093/genetics/161.3.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisne M, Becam AM, Verdiere J, Herbert CJ. A ‘natural’ mutation in Saccharomyces cerevisiae strains derived from S288c affects the complex regulatory gene HAP1 (CYP1). Curr Genet. 1999;36(4):195–200. doi: 10.1007/s002940050490. [DOI] [PubMed] [Google Scholar]
- Gallone B, Steensels J, Prahl T, Soriaga L, Saels V, Herrera-Malaver B, Merlevede A, Roncoroni M, Voordeckers K, Miraglia L, et al. . Domestication and divergence of Saccharomyces cerevisiae beer yeasts. Cell. 2016;166(6):1397–1410.e16. doi: 10.1016/j.cell.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch AP. Yeast genomic expression studies using DNA microarrays. In: Guide to Yeast Genetics and Molecular and Cellular Biology. Heidelberg: Springer; 2002. p. 393–414. [DOI] [PubMed] [Google Scholar]
- Gasch AP, Payseur BA, Pool JE. The power of natural variation for model organism biology. Trends Genet. 2016;32(3):147–154. doi: 10.1016/j.tig.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11(12):4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerke JP, Chen CT, Cohen BA. Natural isolates of Saccharomyces cerevisiae display complex genetic variation in sporulation efficiency. Genetics. 2006;174(2):985–997. doi: 10.1534/genetics.106.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein AC, Berman J. Shift and adapt: the costs and benefits of karyotype variations. Curr Opin Microbiol. 2015;26(1):130–136. doi: 10.1016/j.mib.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein AC, Berman J. Candida albicans genetic background influences mean and heterogeneity of drug responses and genome stability during evolution in fluconazole. mSphere. 2020;5(3):e00480-2. doi: 10.1128/mSphere.00480-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G, Chu AM, Ni L, Connelly C, Riles L, Véronneau S, Dow S, Lucau-Danila A, Anderson K, André B, et al. . Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418(6896):387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- Granados AA, Crane MM, Montano-Gutierrez LF, Tanaka RJ, Voliotis M, Swain PS. Distributing tasks via multiple input pathways increases cellular survival in stress. Elife. 2017;6:e21415. doi: 10.7554/eLife.21415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblum S, Carr R, Borenstein E. Extensive strain-level copy-number variation across human gut microbiome species. Cell. 2015;160(4):583–594. doi: 10.1016/j.cell.2014.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haro R, Garciadeblas B, Rodriguez-Navarro A. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 1991;291(2):189–191. doi: 10.1016/0014-5793(91)81280-L. [DOI] [PubMed] [Google Scholar]
- Hersen P, McClean MN, Mahadevan L, Ramanathan S. Signal processing by the HOG MAP kinase pathway. Proc Natl Acad Sci U S A. 2008;105(20):7165–7170. doi: 10.1073/pnas.0710770105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho CH, Magtanong L, Barker SL, Gresham D, Nishimura S, Natarajan P, Koh JLY, Porter J, Gray CA, Andersen RJ, et al. . A molecular barcoded yeast ORF library enables mode-of-action analysis of bioactive compounds. Nat Biotechnol. 2009;27(4):369–377. doi: 10.1038/nbt.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann S, Krantz M, Nordlander B. Yeast osmoregulation. Methods Enzymol. 2007;428:29–45. doi: 10.1016/S0076-6879(07)28002-4. [DOI] [PubMed] [Google Scholar]
- Hose J, Escalante LE, Clowers KJ, Dutcher HA, Robinson D, Bouriakov V, Coon JJ, Shishkova E, Gasch AP. The genetic basis of aneuploidy tolerance in wild yeast. Elife. 2020;9:e52063. doi: 10.7554/eLife.52063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hose J, Yong CM, Sardi M, Wang Z, Newton MA, Gasch AP. Dosage compensation can buffer copy-number variation in wild yeast. Elife. 2015;4:e05462. doi: 10.7554/eLife.05462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hujoel MLA, Sherman MA, Barton AR, Mukamel RE, Sankaran VG, Terao C, Loh P-R. Influences of rare copy-number variation on human complex traits. Cell. 2022;185(22):4233–4248.e27. doi: 10.1016/j.cell.2022.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanhonou R, Serrano R, Palau RR. A catalytic subunit of the sugar beet protein kinase CK2 is induced by salt stress and increases NaCl tolerance in Saccharomyces cerevisiae. Plant Mol Biol. 2001;47(5):571–579. doi: 10.1023/A:1012227913356. [DOI] [PubMed] [Google Scholar]
- Kishony R, Leibler S. Environmental stresses can alleviate the average deleterious effect of mutations. J Biol. 2003;2(2):14. doi: 10.1186/1475-4924-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvitek DJ, Will JL, Gasch AP. Variations in stress sensitivity and genomic expression in diverse S. cerevisiae isolates. PLoS Genet. 2008;4(10):e1000223. doi: 10.1371/journal.pgen.1000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry CR, Townsend JP, Hartl DL, Cavalieri D. Ecological and evolutionary genomics of Saccharomyces cerevisiae. Mol Ecol. 2006;15(3):575–591. doi: 10.1111/j.1365-294X.2006.02778.x. [DOI] [PubMed] [Google Scholar]
- Lauer S, Gresham D. An evolving view of copy number variants. Curr Genet. 2019;65(6):1287–1295. doi: 10.1007/s00294-019-00980-0. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25(16):2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XC, Peris D, Hittinger CT, Sia EA, Fay JC. Mitochondria-encoded genes contribute to evolution of heat and cold tolerance in yeast. Sci Adv. 2019;5(1):eaav1848. doi: 10.1126/sciadv.aav1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G. The fascinating and secret wild life of the budding yeast S. cerevisiae. Elife. 2015;4:e05835. doi: 10.7554/eLife.05835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liti G, Carter DM, Moses AM, Warringer J, Parts L, James SA, Davey RP, Roberts IN, Burt A, Koufopanou V, et al. . Population genomics of domestic and wild yeasts. Nature. 2009;458(7236):337–341. doi: 10.1038/nature07743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Takekawa M, Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269(5223):554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- Magtanong L, Ho CH, Barker SL, Jiao W, Baryshnikova A, Bahr S, Smith AM, Heisler LE, Choy JS, Kuzmin E, et al. . Dosage suppression genetic interaction networks enhance functional wiring diagrams of the cell. Nat Biotechnol. 2011;29(6):505–511. doi: 10.1038/nbt.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makanae K, Kintaka R, Makino T, Kitano H, Moriya H. Identification of dosage-sensitive genes in Saccharomyces cerevisiae using the genetic tug-of-war method. Genome Res. 2013;23(2):300–311. doi: 10.1101/gr.146662.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel EA, Stuecker TN, Veluvolu M, Gasch AP, Lewis JA. Independent mechanisms for acquired salt tolerance versus growth resumption induced by mild ethanol pretreatment in Saccharomyces cerevisiae. mSphere. 2018;3(6):e00574-18. doi: 10.1128/mSphere.00574-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendizabal I, Pascual-Ahuir A, Serrano R, de Larrinoa IF. Promoter sequences regulated by the calcineurin-activated transcription factor Crz1 in the yeast ENA1 gene. Mol Genet Genomics. 2001;265(5):801–811. doi: 10.1007/s004380100474. [DOI] [PubMed] [Google Scholar]
- Mulet JM, Leube MP, Kron SJ, Rios G, Fink GR, Serrano R. A novel mechanism of ion homeostasis and salt tolerance in yeast: the Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter. Mol Cell Biol. 1999;19(5):3328–3337. doi: 10.1128/MCB.19.5.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rourke SM, Herskowitz I. Unique and redundant roles for HOG MAPK pathway components as revealed by whole-genome expression analysis. Mol Biol Cell. 2004;15(2):532–542. doi: 10.1091/mbc.e03-07-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Ahuir A, Manzanares-Estreder S, Timon-Gomez A, Proft M. Ask yeast how to burn your fats: lessons learned from the metabolic adaptation to salt stress. Curr Genet. 2018;64(1):63–69. doi: 10.1007/s00294-017-0724-5. [DOI] [PubMed] [Google Scholar]
- Peter J, De Chiara M, Friedrich A, Yue JX, Pflieger D, Bergström A, Sigwalt A, Barre B, Freel K, Llored A, et al. . Genome evolution across 1,011 Saccharomyces cerevisiae isolates. Nature. 2018;556(7701):339–344. doi: 10.1038/s41586-018-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrezselyova S, Lopez-Malo M, Canadell D, Roque A, Serra-Cardona A, Marqués MC, Vilaprinyó E, Alves R, Yenush L, Ariño J. Regulation of the Na+/K+-ATPase Ena1 expression by calcineurin/Crz1 under high pH stress: a quantitative study. PLoS One. 2016;11(6):e0158424. doi: 10.1371/journal.pone.0158424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes A, Cadez N, Goncalves P, Sampaio JP. A quasi-domesticate relic hybrid population of Saccharomyces cerevisiae x S. paradoxus adapted to olive brine. Front Genet. 2019;10:449. doi: 10.3389/fgene.2019.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proft M, Serrano R. Repressors and upstream repressing sequences of the stress-regulated ENA1 gene in Saccharomyces cerevisiae: bZIP protein Sko1p confers HOG-dependent osmotic regulation. Mol Cell Biol. 1999;19(1):537–546. doi: 10.1128/MCB.19.1.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putri GH, Anders S, Pyl PT, Pimanda JE, Zanini F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics. 2022;38(10):2943–2945. doi: 10.1093/bioinformatics/btac166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qidwai T. Exploration of copy number variation in genes related to anti-malarial drug resistance in Plasmodium falciparum. Gene. 2020;736:144414. doi: 10.1016/j.gene.2020.144414. [DOI] [PubMed] [Google Scholar]
- Qiu Y, Hassaninasab A, Han GS, Carman GM. Phosphorylation of Dgk1 diacylglycerol kinase by casein kinase II regulates phosphatidic acid production in Saccharomyces cerevisiae. J Biol Chem. 2016;291(51):26455–26467. doi: 10.1074/jbc.M116.763839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios G, Cabedo M, Rull B, Yenush L, Serrano R, Mulet JM. Role of the yeast multidrug transporter Qdr2 in cation homeostasis and the oxidative stress response. FEMS Yeast Res. 2013;13(1):97–106. doi: 10.1111/1567-1364.12013. [DOI] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ, Smyth GK. Edger: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D, Place M, Hose J, Jochem A, Gasch AP. Natural variation in the consequences of gene overexpression and its implications for evolutionary trajectories. Elife. 2021;10:e70564. doi: 10.7554/eLife.70564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Yenush L, Arino J. Regulation of ENA1 Na(+)-ATPase gene expression by the Ppz1 protein phosphatase is mediated by the calcineurin pathway. Eukaryot Cell. 2003;2(5):937–948. doi: 10.1128/EC.2.5.937-948.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha AJ. Java Treeview–extensible visualization of microarray data. Bioinformatics. 2004;20(17):3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- Sardi M, Gasch AP. Genetic background effects in quantitative genetics: gene-by-system interactions. Curr Genet. 2018;64(6):1173–1176. doi: 10.1007/s00294-018-0835-7. [DOI] [PubMed] [Google Scholar]
- Sardi M, Paithane V, Place M, Robinson E, Hose J, Wohlbach DJ, Gasch AP. Genome-wide association across Saccharomyces cerevisiae strains reveals substantial variation in underlying gene requirements for toxin tolerance. PLoS Genet. 2018;14(2):e1007217. doi: 10.1371/journal.pgen.1007217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardi M, Rovinskiy N, Zhang Y, Gasch AP. Leveraging genetic-background effects in Saccharomyces cerevisiae to improve lignocellulosic hydrolysate tolerance. Appl Environ Microbiol. 2016;82(19):5838–5849. doi: 10.1128/AEM.01603-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashkova S, Welkenhuysen N, Hohmann S. Molecular communication: crosstalk between the Snf1 and other signaling pathways. FEMS Yeast Res. 2015;15(4):fov026. doi: 10.1093/femsyr/fov026. [DOI] [PubMed] [Google Scholar]
- Sirr A, Scott AC, Cromie GA, Ludlow CL, Ahyong V, Morgan TS, Gilbert T, Dudley AM. Natural variation in SER1 and ENA6 underlie condition-specific growth defects in Saccharomyces cerevisiae. G3 (Bethesda). 2018;8(1):239–251. doi: 10.1534/g3.117.300392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniegowski PD, Dombrowski PG, Fingerman E. Saccharomyces cerevisiae and Saccharomyces paradoxus coexist in a natural woodland site in North America and display different levels of reproductive isolation from European conspecifics. FEMS Yeast Res. 2002;1:299–306. doi: 10.1111/j.1567-1364.2002.tb00048.x [DOI] [PubMed] [Google Scholar]
- Steenwyk JL, Rokas A. Copy number variation in fungi and its implications for wine yeast genetic diversity and adaptation. Front Microbiol. 2018;9:288. doi: 10.3389/fmicb.2018.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranger BE, Forrest MS, Dunning M, Ingle CE, Beazley C, Thorne N, Redon R, Bird CP, de Grassi A, Lee C, et al. . Relative impact of nucleotide and copy number variation on gene expression phenotypes. Science. 2007;315(5813):848–853. doi: 10.1126/science.1136678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strope PK, Skelly DA, Kozmin SG, Mahadevan G, Stone EA, Stone EA, Magwene PM, Dietrich FS, McCusker JH. The 100-genomes strains, an S. cerevisiae resource that illuminates its natural phenotypic and genotypic variation and emergence as an opportunistic pathogen. Genome Res. 2015;25(5):762–774. doi: 10.1101/gr.185538.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szafraniec K, Borts RH, Korona R. Environmental stress and mutational load in diploid strains of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2001;98(3):1107–1112. doi: 10.1073/pnas.98.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YC, Amon A. Gene copy-number alterations: a cost-benefit analysis. Cell. 2013;152(3):394–405. doi: 10.1016/j.cell.2012.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney KA, Glover CV. Transcriptional regulation of the S. cerevisiae ENA1 gene by casein kinase II. Mol Cell Biochem. 1999;191(1/2):161–167. doi: 10.1023/A:1006893824947. [DOI] [PubMed] [Google Scholar]
- Tumolo JM, Hepowit NL, Joshi SS, MacGurn JA. A Snf1-related nutrient-responsive kinase antagonizes endocytosis in yeast. PLoS Genet. 2020;16(3):e1008677. doi: 10.1371/journal.pgen.1008677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung S, Bakerlee CW, Phillips AM, Nguyen Ba AN, Desai MM. The genetic basis of differential autodiploidization in evolving yeast populations. G3 (Bethesda). 2021;11(8):jkab192. doi: 10.1093/g3journal/jkab192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Thiruvahindrapuram B, Walker S, Wang Z, Hu P, Hu P, Lamoureux S, Wei J, MacDonald JR, Pellecchia G, et al. . A high-resolution copy-number variation resource for clinical and population genetics. Genet Med. 2015;17(9):747–752. doi: 10.1038/gim.2014.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas RC, Garcia-Salcedo R, Tenreiro S, Teixeira MC, Fernandes AR, Ramos J, Sá-Correia I. Saccharomyces cerevisiae multidrug resistance transporter Qdr2 is implicated in potassium uptake, providing a physiological advantage to quinidine-stressed cells. Eukaryot Cell. 2007;6(2):134–142. doi: 10.1128/EC.00290-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A. Energy constraints on the evolution of gene expression. Mol Biol Evol. 2005;22(6):1365–1374. doi: 10.1093/molbev/msi126. [DOI] [PubMed] [Google Scholar]
- Wagner A. On the energy and material cost of gene duplication. In: Dittmar K, Liberles D, editors. Evolution After Gene Duplication. Hoboken, NJ: Wiley-Blackwell; 2011. p. 207–2014. [Google Scholar]
- Warringer J, Zorgo E, Cubillos FA, Zia A, Gjuvsland A, Simpson JT, Forsmark A, Durbin R, Omholt SW, Louis EJ, et al. . Trait variation in yeast is defined by population history. PLoS Genet. 2011;7(6):e1002111. doi: 10.1371/journal.pgen.1002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland J, Nitsche AM, Strayle J, Steiner H, Rudolph HK. The PMR2 gene cluster encodes functionally distinct isoforms of a putative Na+ pump in the yeast plasma membrane. EMBO J. 1995;14(16):3870–3882. doi: 10.1002/j.1460-2075.1995.tb00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkening S, Lin G, Fritsch ES, Tekkedil MM, Anders S, Kuehn R, Nguyen M, Aiyar RS, Proctor M, Sakhanenko NA, et al. . An evaluation of high-throughput approaches to QTL mapping in Saccharomyces cerevisiae. Genetics. 2014;196(3):853–865. doi: 10.1534/genetics.113.160291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will JL, Kim HS, Clarke J, Painter JC, Fay JC, Gasch AP. Incipient balancing selection through adaptive loss of aquaporins in natural Saccharomyces cerevisiae populations. PLoS Genet. 2010;6(4):e1000893. doi: 10.1371/journal.pgen.1000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Hou Y, Bickhart DM, Zhou Y, Hay EHA, Song J, Sonstegard TS, Van Tassell CP, Liu GE. Population-genetic properties of differentiated copy number variations in cattle. Sci Rep. 2016;6(1):23161. doi: 10.1038/srep23161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T, Elbing K, Hohmann S. The pathway by which the yeast protein kinase Snf1p controls acquisition of sodium tolerance is different from that mediating glucose regulation. Microbiology (Reading). 2008;154(9):2814–2826. doi: 10.1099/mic.0.2008/020149-0. [DOI] [PubMed] [Google Scholar]
- Yenush L. Potassium and sodium transport in yeast. Adv Exp Med Biol. 2016;892:187–228. doi: 10.1007/978-3-319-25304-6_8. [DOI] [PubMed] [Google Scholar]
- Young MJ, Court DA. Effects of the S288c genetic background and common auxotrophic markers on mitochondrial DNA function in Saccharomyces cerevisiae. Yeast. 2008;25(12):903–912. doi: 10.1002/yea.1644. [DOI] [PubMed] [Google Scholar]
- Zarin T, Moses AM. Insights into molecular evolution from yeast genomics. Yeast. 2014;31(7):233–241. doi: 10.1002/yea.3018. [DOI] [PubMed] [Google Scholar]
- Zmienko A, Samelak A, Kozlowski P, Figlerowicz M. Copy number polymorphism in plant genomes. Theor Appl Genet. 2014;127(1):1–18. doi: 10.1007/s00122-013-2177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Barcode sequencing data are available in the NIH GEO database under accession number GSE226247. RNA sequencing data are available in the NIH GEO database under accession number GSE226246.
Supplemental material available at G3 online.