Abstract
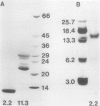
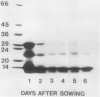
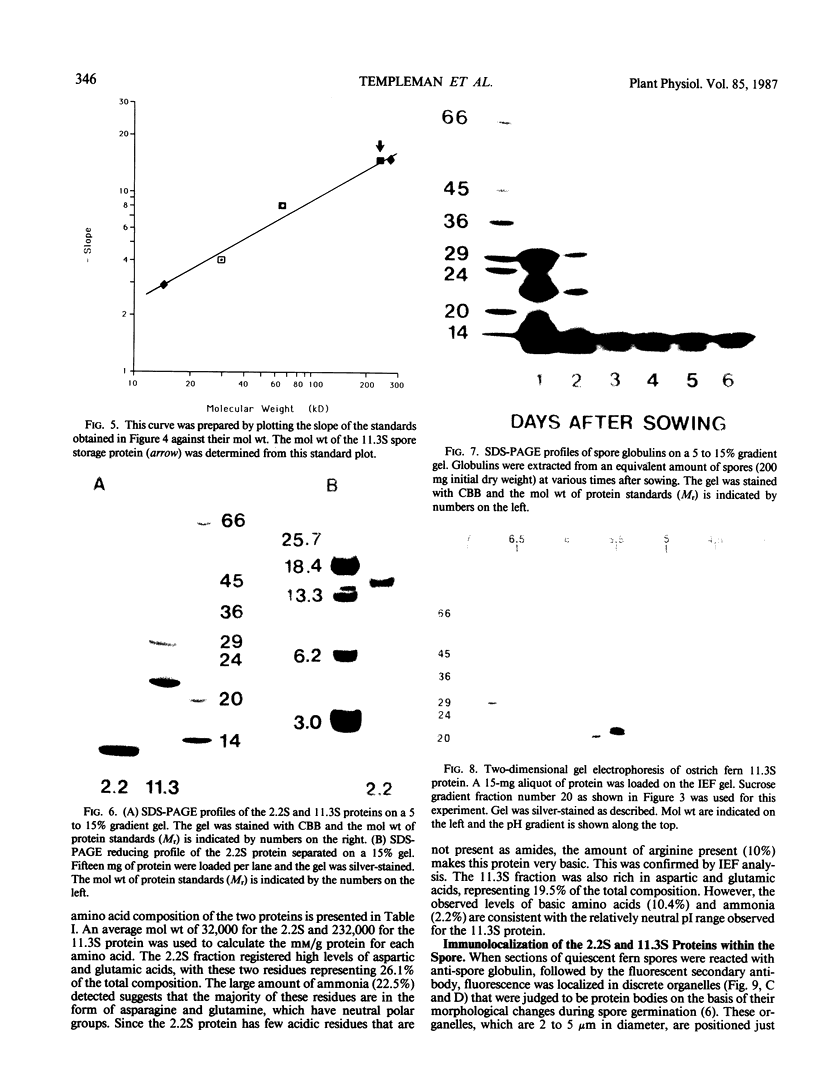
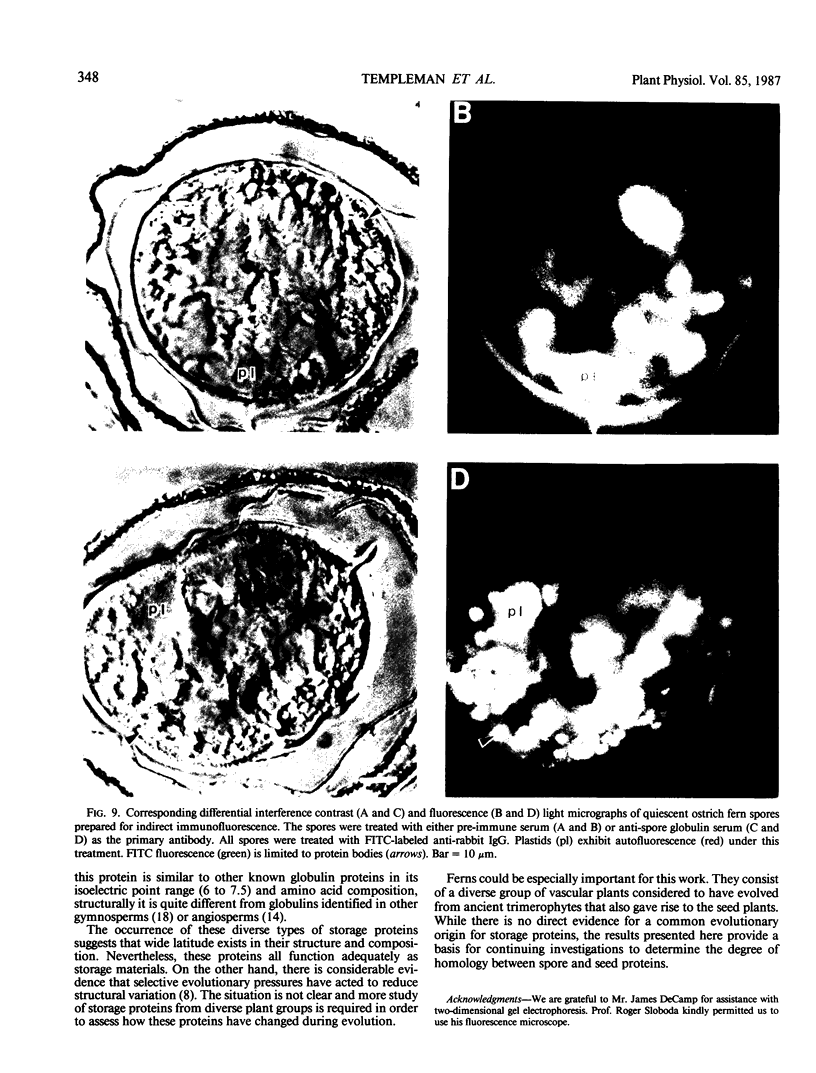
Two globulin storage proteins have been identified in spores of the ostrich fern, Matteuccia struthiopteris (L.) Todaro. The two proteins comprise a significant amount of the total spore protein, are predominantly salt-soluble, and can be extracted by other solvents to a limited extent. The large 11.3 Svedberg unit (S) globulin is composed of five polypeptides with molecular weights of 21,000, 22,000, 24,000, 28,000 and 30,000. Each polypeptide has several isoelectric point (pI) variants between pH 5 and 7. The small 2.2S storage protein has a pI > 10.5 and is composed of at least two major polypeptides of 6,000 and 14,000 Mr. The amino acid composition of both storage proteins reveals that the 11.3S protein is particularly rich in aspartic and glutamic acid, while the 2.2S protein has few acidic amino acids. During imbibition and germination the globulin fraction declines rapidly, with a corresponding degradation of individual polypeptides of each protein. Polyclonal antibodies against each of the two proteins were produced and used for immunolocalization to determine the site of storage protein deposition within the quiescent spore. The proteins were sequestered in protein bodies of 2 to 10 micrometers, that are morphologically similar to those found in the seeds of flowering plants. The results suggest that spore globulins are biochemically similar to seed globulins, especially those found in some cruciferous seeds.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen H. P., Demaggio A. E. Biochemistry of fern spore germination: protease activity in ostrich fern spores. Plant Physiol. 1986 Apr;80(4):992–996. doi: 10.1104/pp.80.4.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S., Goodchild D. J. Post-embedding immunolabelling. Some effects of tissue preparation on the antigenicity of plant proteins. Eur J Cell Biol. 1982 Oct;28(2):251–256. [PubMed] [Google Scholar]
- Crouch M. L., Tenbarge K. M., Simon A. E., Ferl R. cDNA clones for Brassica napus seed storage proteins: evidence from nucleotide sequence analysis that both subunits of napin are cleaved from a precursor polypeptide. J Mol Appl Genet. 1983;2(3):273–283. [PubMed] [Google Scholar]
- Doyle J. J., Schuler M. A., Godette W. D., Zenger V., Beachy R. N., Slightom J. L. The glycosylated seed storage proteins of Glycine max and Phaseolus vulgaris. Structural homologies of genes and proteins. J Biol Chem. 1986 Jul 15;261(20):9228–9238. [PubMed] [Google Scholar]
- Fechner A., Schraudolf H. Aphidicolin Inhibition of DNA Synthesis and Germination in Spores of Anemia phyllitidis L. Sw. Plant Physiol. 1986 Jun;81(2):714–716. doi: 10.1104/pp.81.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliss E. R., Setlow P. Genes for Bacillus megaterium small, acid-soluble spore proteins: nucleotide sequence of two genes and their expression during sporulation. Gene. 1985;35(1-2):151–157. doi: 10.1016/0378-1119(85)90167-2. [DOI] [PubMed] [Google Scholar]
- Kreis M., Forde B. G., Rahman S., Miflin B. J., Shewry P. R. Molecular evolution of the seed storage proteins of barley, rye and wheat. J Mol Biol. 1985 Jun 5;183(3):499–502. doi: 10.1016/0022-2836(85)90017-8. [DOI] [PubMed] [Google Scholar]
- Langridge P., Moran G. F., Brown A. H. Biochemical genetics of some seed proteins of Pinus radiata. Biochem Genet. 1981 Jun;19(5-6):585–597. doi: 10.1007/BF00484628. [DOI] [PubMed] [Google Scholar]
- Laroche-Raynal M., Delseny M. Identification and characterization of the mRNA for major storage proteins from radish. Eur J Biochem. 1986 Jun 2;157(2):321–327. doi: 10.1111/j.1432-1033.1986.tb09671.x. [DOI] [PubMed] [Google Scholar]
- Laroche M., Aspart L., Delseny M., Penon P. Characterization of Radish (Raphanus sativus) Storage Proteins. Plant Physiol. 1984 Mar;74(3):487–493. doi: 10.1104/pp.74.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Robert L. S., Adeli K., Altosaar I. Homology among 3S and 7S Globulins from Cereals and Pea. Plant Physiol. 1985 Aug;78(4):812–816. doi: 10.1104/pp.78.4.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWARD F. C., LYNDON R. F., BARBER J. T. ACRYLAMIDE GEL ELECTROPHORESIS OF SOLUBLE PLANT PROTEINS: A STUDY ON PEA SEEDLINGS IN RELATION TO DEVELOPMENT. Am J Bot. 1965 Feb;52:155–164. [PubMed] [Google Scholar]
- Simon R. D. Measurement of the cyanophycin granule polypeptide contained in the blue-green alga Anabaena cylindrica. J Bacteriol. 1973 Jun;114(3):1213–1216. doi: 10.1128/jb.114.3.1213-1216.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. D., Weathers P. Determination of the structure of the novel polypeptide containing aspartic acid and arginine which is found in Cyanobacteria. Biochim Biophys Acta. 1976 Jan 20;420(1):165–176. doi: 10.1016/0005-2795(76)90355-x. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Kornberg A. Biochemical studies of bacterial sporulation and germaination. VII. Protein turnover during sporulation of Bacillus subtilis. J Biol Chem. 1968 Sep 10;243(17):4600–4605. [PubMed] [Google Scholar]