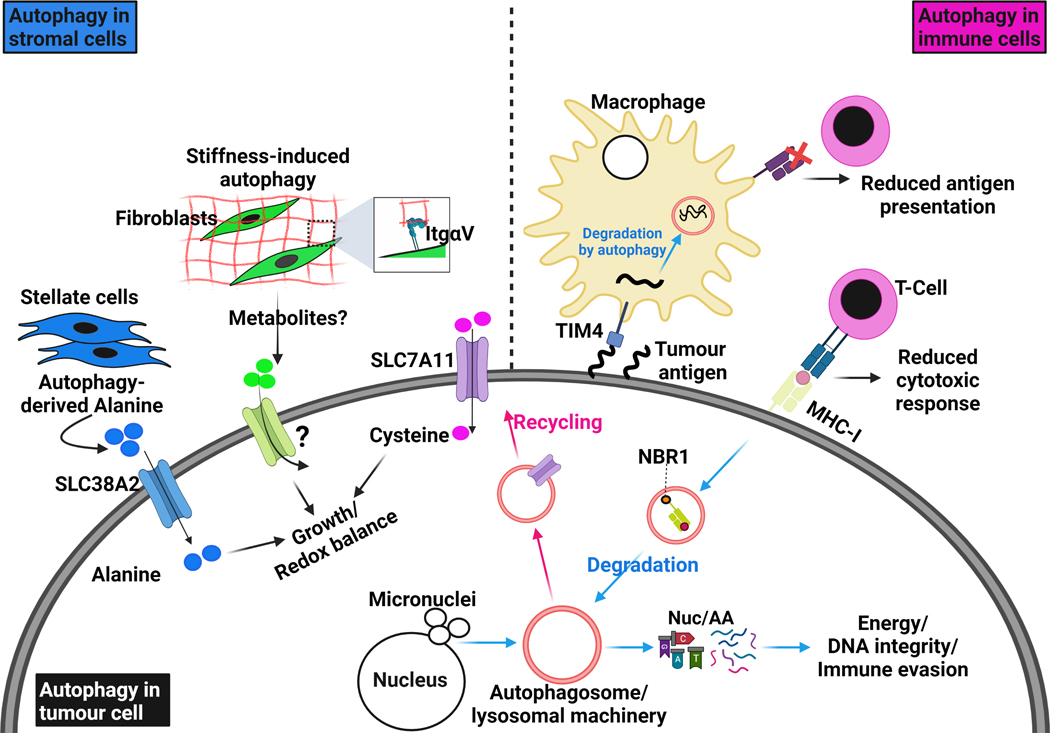
Figure 3. Autophagy in the tumour and surrounding microenvironment.
Autophagy is involved in complex crosstalk between tumour cells and their surrounding microenvironment. Cell-autonomous autophagy in tumour cells is important to relieve replication stress and maintain DNA stability by removing damaged chromosomes and micronuclei, which are the result of aberrant proliferation. It also degrades and recycles essential components for redox homeostasis like the cystine transporter SLC7A11. As energy demands in tumour cells are progressively increasing, they produce factors that activate the stromal component in their microenvironment. Tumour-stimulated stromal cells activate autophagy to release amino acids, such as alanine, which in turn are up-taken by tumour cells through specific transporters, like SLC38A2, to fuel their metabolic networks. Integrin-mediated interaction with stiff extracellular matrix (ECM) directly activates autophagy in stromal cells, such as fibroblasts and stellate cells, and provides tumour cells with growth advantages. More studies are still needed to understand whether ECM stiffness modulates nutrients release from stromal cells. Therefore, autophagy in tumour cells and stromal cells provides the former with sufficient amino acids and nucleotide pools to survive intense episodes of stress. The degradation of surface proteins such as MHC-I by autophagy allows tumour cells to escape anti-tumor immunity; MHC-I degradation and delivery to autophagosomes is dependent on the cargo NBR1. Interestingly, autophagy in immune cells can also sense and degrade tumour antigens through TIM4 and reduce their presentation to cytotoxic T-cells. All these examples illustrate the autophagy-dependent crosstalk that exists between tumour cells and their surrounding microenvironment.