Post-transplant lymphoproliferative disorders (PTLD) are a heterogeneous group of conditions that involve uncontrolled proliferation of lymphoid cells developing as a consequence of extrinsic immunosuppression after solid organ or hematopoietic stem cell transplantation.1 The 2022 World Health Organization (WHO) classification distinguishes lymphomas from other proliferations arising in the setting of immune deficiency and further categorizes them as in immunocompetent patients, the most frequent being diffuse large B-cell lymphoma (DLBCL).
Based on several phase II trials,2,3 rituximab-based therapy following reduction of immunosuppressive therapy (RIS) is now standard of care for most CD20+ PTLD. Sequential therapy (4 weekly courses of rituximab either completed by 4 more courses of rituximab in responders [complete remission [CR] and low-risk partial remission [PR] group: 25-26%] or by 4 cycles of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone every 21 days [R-CHOP-21] in patients not responding to rituximab monotherapy sufficiently [74-75%]) showed encouraging results for CD20+ B-cell PTLD with 7-8% treatment-related-mortality (TRM), 2-year progression-free survival (PFS) of 56-67% and 2-year overall survival (OS) of 68-72%.4,5 Current PTLD guidelines encourage sequential therapy in CD20+ polymorphic and monomorphic DLBCL-type PTLD that fail to respond to upfront RIS or if RIS is not feasible.6
Burkitt lymphoma (BL) PTLD (BL-PTLD) is a rare subtype of post-transplant lymphoma representing less than 10% of adult PTLD.7 Its immune-competent counterpart is characterized by a highly aggressive clinical course, frequent involvement of bone marrow (BM) and central nervous system (CNS), and is biologically hallmarked by MYC gene translocation to an immunoglobulin locus. Due to the reduced number of BL-PTLD cases, their clinical characteristics, outcome, and most adequate therapeutic options are not well established.8–10 We conducted a multicenter retrospective study in order to better characterize these elements.
Our study included adult patients diagnosed with BL-PTLD between 1989 and 2022 who were identified using the French registry of PTLD after kidney transplantation, the K-Virogref network, the Lymphopath database and the prospective German PTLD registry. After the review of medical and pathological records, 55 patients treated in 20 French and eight German centers were included. The study was approved by national (CNIL 913611) and local (CPP Ile-De-France PP 13-022) ethics committees. Diagnosis of BL-PTLD followed criteria from the 2022 revised edition of the WHO classification,11 taking into account morphology, immunohistochemistry (CD20, CD10, BCL2, BCL6, MUM-1, Ki67 data) and cytogenetics (C-MYC, BCL2, BCL6 in fluorescence in situ hybridization [FISH] and/or karyotype). Association with Eppstein Barr virus (EBV) was assessed by in situ hybridization for Epstein Barr-encoded small RNA (EBER). Among different therapeutic options, the most frequently used chemotherapies were: CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone), EPOCH (etoposide, doxorubicin, vincristine, cyclophosphamide, prednisone), COPADEM (vincristine, methotrexate, cyclophosphamide, doxorubicin, prednisone 60 mg/m2) and hyperCVAD (hyperfractioned cyclophosphamide, vincristine, doxorubicin, dexamethasone). Response to treatment followed standard international criteria.12,13 Subgroup comparisons were performed using Χ2 of Fisher’s exact test for categorical variables, and Student’s t-test for continuous variables. Time-to-event outcomes were estimated using Kaplan-Meier curves, and were compared using the logrank test. The level of significance in all analyses was set at P<0.05. Statistical analysis was performed using R Studio 2021.09.1.
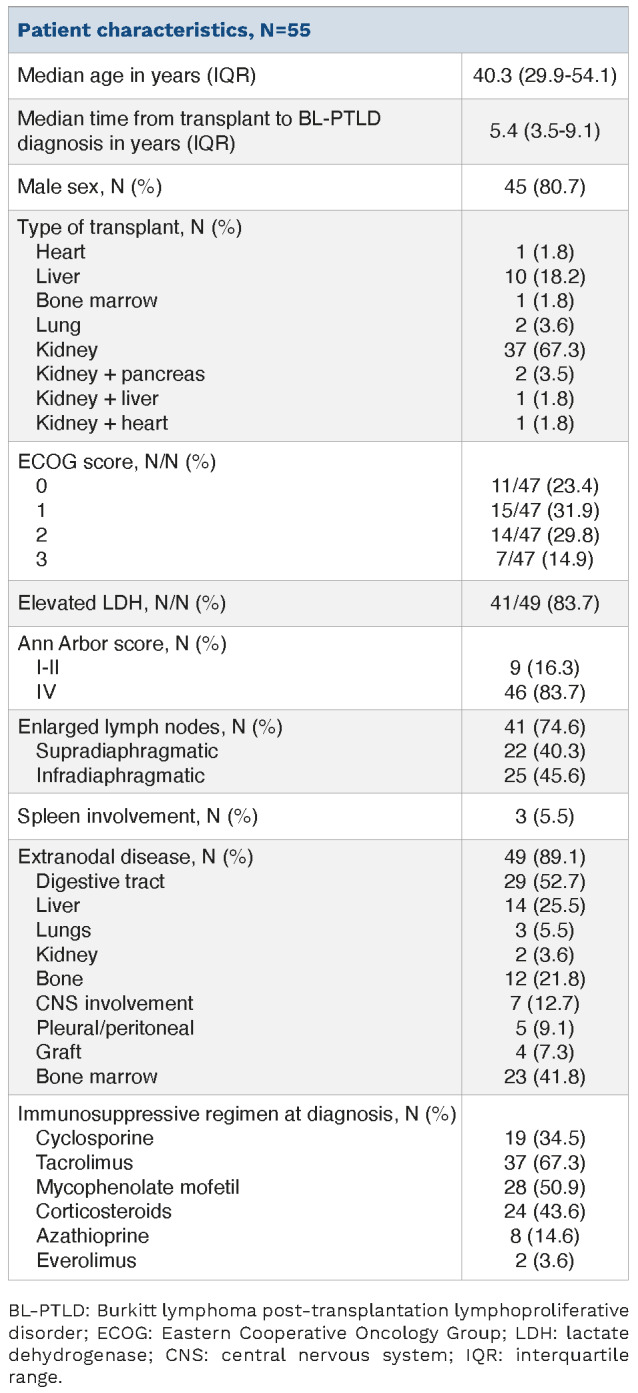
The study population comprised 55 patients, with a median age at diagnosis of 40.3 years and the most frequently transplanted organs were kidney (n=37) and liver (n=10) (Table 1). Median time from transplant to BL-PTLD diagnosis was 5.4 years (range, 0.2-24). Most patients presented with an aggressive and advanced stage of disease at diagnosis: Eastern Cooperative Oncology Group (ECOG) score ≥2 in 44.6% (n=21/47 cases), elevated lactate dehydrogenase (LDH) in 84% (n=41/49), Ann Arbor stage IV in 84% (n=46/55) patients. Extranodal disease was frequent (n=49, 89%), with the digestive tract being the most frequently involved organ (n=29, 53%). CNS infiltration was observed in seven patients (13%). BL-PTLD tumor samples were all CD20+, CD10+, BCL2-, expressed Ki67 in more than 90% of cells and harbored MYC rearrangement detected by FISH. EBER was positive in 12 of 29 samples (41%) and LMP was positive in one of nine (11%).
Fifty-three patients received curative-intended therapy while two patients received supportive care only. RIS never was proposed as a unique initial strategy but was performed in 42 of 53 (79%) patients. Fourteen of 53 (25%) patients were treated according to sequential strategies. These patients received up to four weekly courses of rituximab followed either by: (i) rituximab consolidation (4 3-weekly courses) in a single case of CR (1/14, 7%) or (ii) subsequent chemo(immuno)therapy (C(I)T) (n=13/14, 93%): CHOP (n=2), R-CHOP (n=9, including one with concomitant high dose methotrexate [MTX]), R-COPADEM followed by R-CYM (n=1), unknown (n=1). Seven (13%) patients received frontline polychemotherapy (CT) (COPADEM [n=6] and CHOP [n=1]) and 32 (60%) were treated with CIT (R-COPADEM [n=12], R-CHOP [n=13, 7 without MTX, 6 with sequential or concomitant MTX], R-EPOCH [n=5], R-hyperCVAD [n=1], rituximab with whole brain radiotherapy [WBRT] and 90Y-ibritumomab tiuxetan [n=1]) (Online Supplementary Figure S1). There was no significant difference between patients who had received sequential therapy or frontline CT/CIT, except that patients with CNS involvement were all treated with CT/CIT and those treated with sequential therapy had a lower ECOG score (Online Supplementary Table S1).
Table 1.
Baseline characteristics of Burkitt lymphoma posttransplantation lymphoproliferative disorder patients.
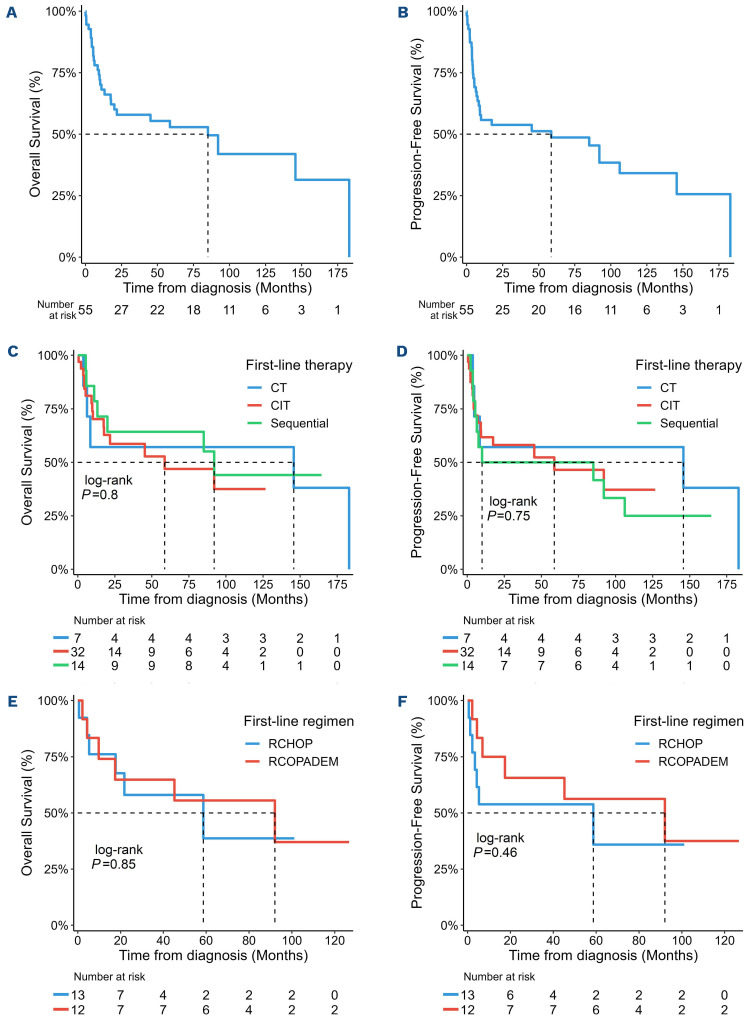
Median follow-up was 7.5 years (95% confidence interval [CI]: 5.8-10.5). Median OS and PFS were 7.1 (95% CI: 1.7-not applicable [NA])and 4.9 years (95% CI: 0.7-NA) respectively (Figure 1A, B). Thirty-eight patients (70%) attained CR after first-line treatment, including four of seven (57%) who received CT, 25 of 32 (78%) after CIT, and nine of 14 (64%) after completing sequential treatment. With sequential treatment, the overall response rate (CR + PR) to four doses of rituximab was 21% (1 CR, 2 PR). Among patients receiving frontline CIT, CR rates were 61%, 100% and 83% for patients treated with R-CHOP, R-EPOCH and R-COPADEM respectively, without significant statistical difference (P=0.26). Relapses occurred in four patients who had attained CR after a median of 6 months. Two had received upfront CIT and two sequential therapy. Out of these, only a patient relapsing after sequential therapy containing R-CHOP was successfully rescued by another CIT regimen. Three-year OS and PFS for the whole cohort were 58% (95% CI: 45.8-73.1) and 54% (95% CI: 41.9-68.9), respectively. For patients receiving upfront CIT, 3-year OS and PFS were 59% (95% CI: 42.9-80.1) and 58% (95% CI: 43-78.5). For patients receiving sequential therapy, 3-year OS and PFS were 64% (95% CI: 43.5-95.0) and 50% (95% CI: 29.6-84.4) (Figure 1C, D). OS and PFS after CIT and sequential therapy were not statistically different. There was no significant difference in PFS or OS between patients receiving upfront R-CHOP or R-COPADEM regimens (Figure 1E, F). Patients with CNS involvement (n=7) were treated with intrathecal (n=3) or high-dose intravenous MTX (n=2) or both (n=1) or WBRT (n=1) and five of them achieved CR. Twenty-two of 48 (46%) patients without CNS involvement received CNS prophylaxis during first-line therapy (either by intrathecal or systemic chemotherapy containing cytarabine or MTX). CNS prophylaxis had no impact on OS or PFS (data not shown).
Twenty-nine deaths occurred during follow-up mainly due to progressive disease (n=11, 38%). Treatment-related deaths (n=7, 24%; including sepsis, n=6; cerebral hemorrhage, n=1) occurred during frontline CIT treatment in five patients and in the relapse setting in two patients (Online Supplementary Table S2).
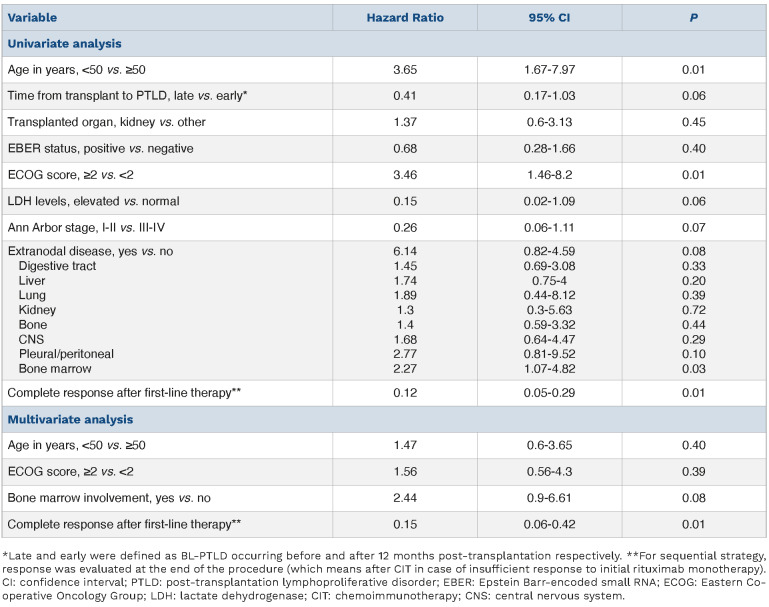
Univariate analysis showed that age over 50 years, ECOG score and BM involvement were significant predictors for poorer OS whereas complete response to first-line therapy was associated with longer survival and was the only variable retaining significance in multivariate analysis (Table 2). Complete response to first-line therapy was not significantly associated with other variables (i.e., age, transplanted organ, EBER, ECOG score, LDH levels, stage, BM or CNS involvement, or type of treatment).
Figure 1.
Survival for the whole cohort, according to the type of therapy and according to the type of chemoimmunotherapy. (A) Overall survival (OS) and (B) progression free-survival (PFS) for the whole Burkitt lymphoma post-transplantation lymphoproliferative disorder (BL-PTLD) cohort. The dashed lines indicate the respective times of median survival. (C) OS and (D) PFS by type of therapies used: chemotherapy (CT, N=7) (blue line), chemoimmunotherapy (CIT, N=32) (red line) and sequential therapy (sequential, N=14) (green line). (E) OS, (F) PFS according to the type of CIT regimen: R-CHOP (N=13) (blue line), R-COPADEM (N=12) (red line). R-CHOP: cyclophosphamide, doxorubicin, prednisone, rituximab and vincristine; R-COPADEM: rituximab, cyclophosphamide, vincristine, prednisone, doxorubicin, and methotrexate.
To our knowledge, this is the largest retrospective series of BL-PTLD to date. BL-PTLD is a late-onset PTLD (like -PTLD5) with an aggressive presentation, frequent advanced disease, altered performance status and CNS involvement. In our series, EBV association (41%) appears to be similar to what is observed in DLBCL-PTLD (around 50%) and differs from previous series of BL-PTLD,7,9 in keeping with publications suggesting that late-onset PTLD are more frequently EBV-negative.1 Reflecting the lack of consensus, our retrospective BL-PTLD cohort used a variety of therapeutic approaches, ranging from high-dose chemotherapy and R-CHOP-based regimens to rituximab monotherapy.
In a sequential strategy setting, the CR rate after the first four courses of rituximab monotherapy was very low (7%), but subsequent CIT provided disease control similar to the frontline CIT strategy (similar CR rate, OS and PFS). Although rituximab monotherapy does not appear to be a valuable option in BL-PTLD, sequential therapy could be discussed to avoid early complications associated with up-front C(I)T, considering the relatively high TRM observed in this series (13%). R-CHOP-based regimens yielded similar CR rates, PFS and OS compared to more dose-intensive regimens such as R-COPADEM and represent a valuable option in BL-PTLD, even with only four cycles as part of sequential therapy. In univariate analyses, age, ECOG score and BM involvement were significantly associated with poorer OS as in the immunocompetent BL cohort from which the BL-IPI was derived,14 while complete response to first-line therapy was the strongest prognostic factor for longer OS in univariate and multivariate analyses. One of the main limitations of our work is its retrospective nature and the use of different sources for patient selection. The fact that the majority of cases occurred after kidney transplantation is probably partly related to selection bias, but also to the fact that kidney transplantation is by far the most frequent transplantation in adults. This may partly explain the contrast between our data and a recently published pediatric cohort of BL-PTLD,15 in which most patients had received heart or liver transplantation, although other elements (such as higher EBV association) may suggest a different disease biology.
Table 2.
Predictors of overall survival (Cox model, univariate and multivariate).
In conclusion, our study confirms that BL-PTLD is an aggressive, late-onset PTLD with frequent extranodal and CNS localization. Although initial rituximab monotherapy is not as effective as for non-Burkitt PTLD, sequential strategy was as effective as frontline CIT regimens among which R-CHOP and more dose intensive regimens showed similar outcomes.
Supplementary Material
Acknowledgments
The authors thank Camille Laurent, Nadia Amara, Elise Chapiro, Laura Rozalska, Patricia Brugel and Hervé Sartelet for providing histological and cytogenetic information.
Funding Statement
Funding: This study was supported by grants from INCA-DGOS-INSERM-12560 (SiRIC CURAMUS is financially supported by the French National Cancer Institute, the French Ministry of Solidarity and Health and INSERM with financial support from ITMO Cancer AVIESAN).
References
- 1.Dharnidharka VR, Webster AC, Martinez OM, et al. Post-transplant lymphoproliferative disorders. Nat Rev Dis Primers. 2016;2:15088. [DOI] [PubMed] [Google Scholar]
- 2.Choquet S, Leblond V, Herbrecht R, et al. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study. Blood. 2006;107(8):3053-3057. [DOI] [PubMed] [Google Scholar]
- 3.Trappe R, Oertel S, Leblond V, et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13(2):196-206. [DOI] [PubMed] [Google Scholar]
- 4.Trappe RU, Dierickx D, Zimmermann H, et al. Response to rituximab induction is a predictive marker in B-cell post-transplant lymphoproliferative disorder and allows successful stratification into rituximab or R-CHOP consolidation in an international, prospective, multicenter phase II trial. J Clin Oncol. 2017;35(5):536-543. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann H, Koenecke C, Dreyling MH, et al. Modified risk-stratified sequential treatment (subcutaneous rituximab with or without chemotherapy) in B-cell post-transplant lymphoproliferative disorder (PTLD) after solid organ transplantation (SOT): the prospective multicentre phase II PTLD-2 trial. Leukemia. 2022;36(10):2468-2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah N, Eyre TA, Tucker D, et al. Front-line management of post-transplantation lymphoproliferative disorder in adult solid organ recipient patients - a British Society for Haematology guideline. Br J Haematol. 2021;193(4):727-740. [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann H, Reinke P, Neuhaus R, et al. Burkitt posttransplantation lymphoma in adult solid organ transplant recipients: sequential immunochemotherapy with rituximab (R) followed by cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or R-CHOP is safe and effective in an analysis of 8 patients. Cancer. 2012;118(19):4715-4724. [DOI] [PubMed] [Google Scholar]
- 8.Xicoy B, Ribera J-M, Esteve J, et al. Post-transplant Burkitt’s leukemia or lymphoma. Study of five cases treated with specific intensive therapy (PETHEMA ALL-3/97 trial). Leuk Lymphoma. 2003;44(9):1541-1543. [DOI] [PubMed] [Google Scholar]
- 9.Bobillo S, Abrisqueta P, Sánchez-González B, et al. Posttransplant monomorphic Burkitt’s lymphoma: clinical characteristics and outcome of a multicenter series. Ann Hematol. 2018;97(12):2417-2424. [DOI] [PubMed] [Google Scholar]
- 10.Dierickx D, Tousseyn T, Gheysens O. How I treat posttransplant lymphoproliferative disorders. Blood. 2015;126(20):2274-2283. [DOI] [PubMed] [Google Scholar]
- 11.Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720-1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Younes A, Hilden P, Coiffier B, et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol. 2017;28(7):1436-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. [DOI] [PubMed] [Google Scholar]
- 14.Olszewski AJ, Jakobsen LH, Collins GP, et al. Burkitt lymphoma International Prognostic Index. J Clin Oncol. 2021;39(10):1129-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afify Z, Orjuela-Grimm M, Smith CM, et al. Burkitt lymphoma after solid-organ transplant: treatment and outcomes in the paediatric PTLD collaborative. Br J Haematol. 2023;200(3):297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.